Pethia ticto ( Hamilton, 1822 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3964.4.1 |
|
publication LSID |
lsid:zoobank.org:pub:5982F525-B457-4B18-BE95-7D4E9E7E31EC |
|
DOI |
https://doi.org/10.5281/zenodo.5664390 |
|
persistent identifier |
https://treatment.plazi.org/id/9D112B52-814E-4947-FF46-BDEBFE3800ED |
|
treatment provided by |
Plazi |
|
scientific name |
Pethia ticto ( Hamilton, 1822 ) |
| status |
|
Pethia ticto ( Hamilton, 1822) View in CoL
( Figures 1 View FIGURE 1 A, B, 2, 3)
Cyprinus ticto Hamilton, 1822: 314 View in CoL , plate VIII fig.87
Systomus ticto ( Hamilton, 1822) View in CoL : M’Clelland 1839: 382, plate 8 f. 87; Bleeker 1844: 260 Barbus ticto ( Hamilton, 1822) View in CoL : Günther 1868: 153; Day 1878: 576; Day 1871: 290; Hora 1939: 263 Puntius ticto ( Hamilton, 1822) View in CoL : Motwani & David 1957: 11; Menon 1952: 268; Menon 1999: 103 Pethia ticto ( Hamilton, 1822) View in CoL : Pethiyagoda et al. 2012: 81
Material examined. 5 ex., BNHS FWF 127 to 131, Beri Baor, Ramnagar, Kolkata, West Bengal, India (22° 54' 32"N, 88° 51' 14" E, 5 m a.s.l.), coll. U. Katwate, R. Raghavan and N. Dahanukar, 6 June 2014; 4 ex., WILD-15- PIS-145 to 148, Beri Baor, Ramnagar, Kolkata, West Bengal, India (22° 54' 32"N, 88° 51' 14" E, 5 m a.s.l.), coll. U. Katwate, R. Raghavan and N. Dahanukar, 6 June 2014; 2 ex., ZSI-WRC P 4360, Beri Baor, Ramnagar, Kolkata, West Bengal, India (22° 54' 32"N, 88° 51' 14" E, 5 m a.s.l.), coll. U. Katwate, R. Raghavan and N. Dahanukar, 6 June 2014; 2 ex., DABFUK/FI/223, Beri Baor, Ramnagar, Kolkata, West Bengal, India (22° 54' 32"N, 88° 51' 14" E, 5 m a.s.l.), coll. U. Katwate, R. Raghavan and N. Dahanukar, 6 June 2014; 1 ex., unregistered, Dhaka, Bangladesh, coll. Muntasir Akash, 24 March 2015 (only photograph examined).
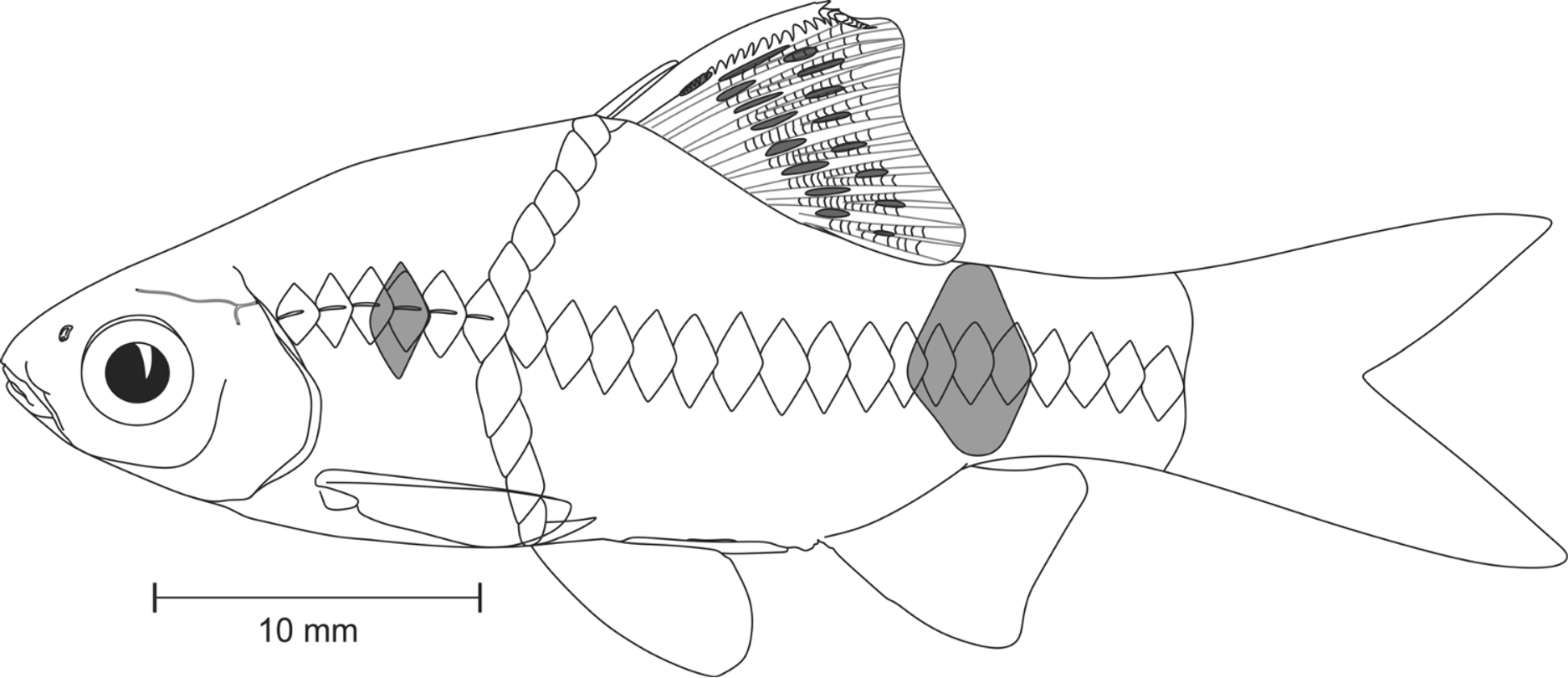
Diagnosis. Pethia ticto can be distinguished from all other species of Pethia by the combination of the following characters: barbels absent; lateral line incomplete, with 6–12 pored scales; 23–26 scales in lateral-scale row; predorsal scales 9; scales in transverse series ½4/1/3½–4; dorsal fin originating after pelvic-fin origin; gill rakers 7 on first ceratobranchial; 17 abdominal including 4 weberian and 13 caudal vertebrae including posteriormost compound centrum (i.e. 4+13+13); banding pattern includes a small black humeral spot covering third and fourth lateral-line scales; a prominent spot on the caudal peduncle on scales 16–19 of lateral scale row; and dorsal fin with 2 oblique rows of black spots.
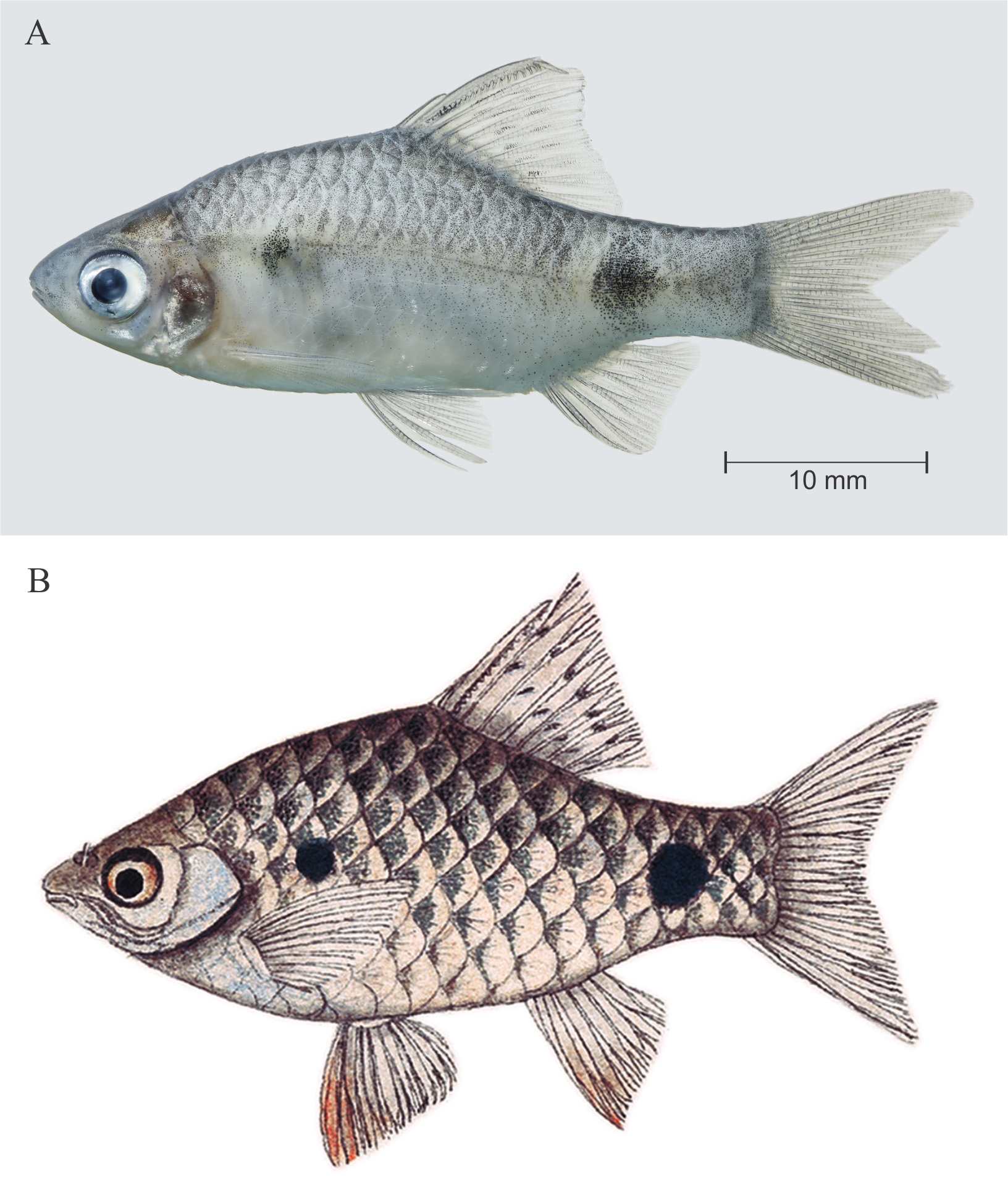
Description. Illustration and photograph of topotypes are provided in Figures 1 View FIGURE 1 , 2 View FIGURE 2 A–B, 3A–B. Morphometric and meristic data are provided in Table 1 View TABLE 1 .
Body short, deep, its length 2.8 to 3.2 times depth; compressed laterally; predorsal contour slightly convex, rising gradually up to dorsal-fin origin, thereafter sloping down towards hypural notch in a sharp concave slope. Ventral profile convex up to base of pectoral fin, then almost straight up to anterior extremity of anal-fin base, sloping gradually towards hypural notch. Caudal peduncle broad, short, its length 1.2–1.6 times its depth.
Head small, laterally compressed. Snout pointed, smooth, its length less than eye diameter. Eyes large, midlaterally positioned, closer to snout tip than margin of operculum, diameter 0.8–1.3 interorbital width. Mouth small, terminal, ventrally ‘U’ shaped, angle of gape not reaching to vertical from anterior margin of eye. Upper and lower lips thin. Barbels absent.
Dorsal fin originating posterior to pelvic-fin origin, closer to tip of snout than to base of caudal fin, its distal margin concave, height 0.9–1.1 times head length. Dorsal fin with 4 unbranched (including 3 supernumerary rays) and 8 branched rays, last unbranched ray strong, its proximal half smooth, densely serrated posteriorly with 11 (1), 13 (3), 14 (1), 15 (1), 16 (2), 17 (1), 18 (1), 19 (2) or 21 (1) serrae on its distal half, 2 serrae on apex. Pectoral fin with one unbranched and 12 (3), 13 (3), 14 (1) or 15 (6) branched rays, its tip rounded, extending beyond pelvic-fin origin ( Figure 1 View FIGURE 1 , 2 View FIGURE 2 A, 3A & B). Pelvic fin with one unbranched and 7 branched rays, its tip rounded, not reaching vent when adpressed. Anal fin with 3 unbranched (including 2 supernumerary) and 5 branched rays, its distal margin concave, with rounded angles. Caudal fin forked, lobes representing more than half of fin length, tips pointed. Principal caudal-fin rays dorsally 9, ventrally 8; procurrent rays dorsally 7, ventrally 6.
Lateral line incomplete with 6 (1), 7 (3), 9 (5), 11 (3) or 12 (1) pored scales. Lateral-scale row with 23 (3), 24 (1), 25 (6) or 26 (3) scales. Scales in transverse series ½4/1/3½ (3) or ½4/1/4 (10); predorsal scales 9; prepelvic scales 10 (9), 11 (2) or 12 (2); preanal scales 16 (4), 17 (8) or 19 (1); circumpeduncular scales 12. Pelvic axillary scale present, reaching to one-fourth adpressed pelvic-fin length.
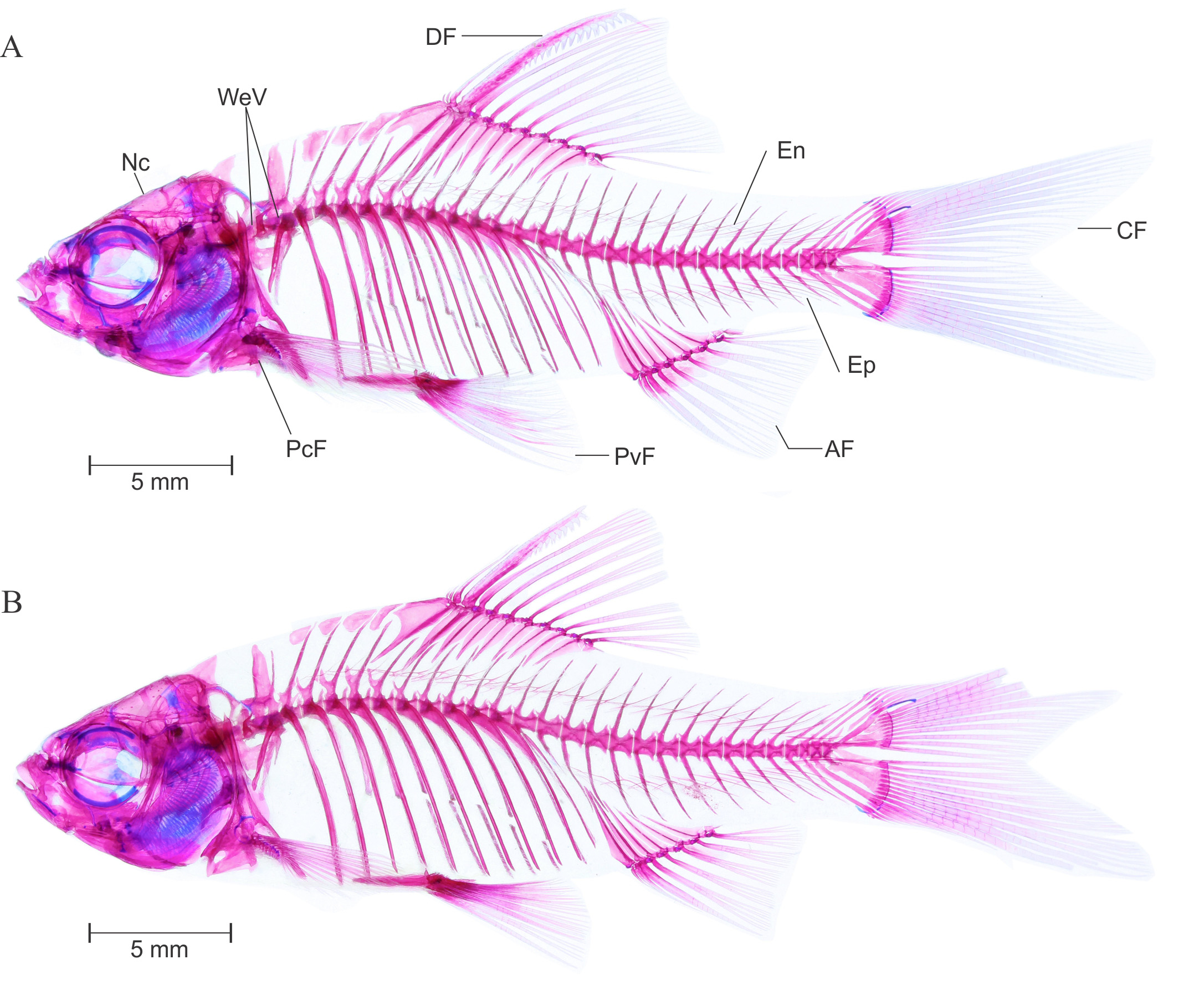
Osteology. Complete lateral view of the cleared and stained specimens of P. t i c t o (BNHS FWF 130, 131) are provided in Figure 4 View FIGURE 4 A and B, respectively.
Neurocranium: Dorsal, lateral and ventral view of neurocranium provided in Figure 5A–C. In general appearance, it is broad and wide at otic region, and narrow and slopes sloping dorsally towards ethmoid region. Ethmoid region is composed of mesethmoid, prethmoid, ethmoid, kinethmoid (Figure 5A), vomer and large winged lateral ethmoid. Ethmoid region slopes down anteroventrally, meeting the triangular mesethmoid, which is placed between lateral wings of considerably cartilaginous ethmoid portion. Lateral ethmoid contributes large portion in the ethmoid complex, clearly visible, lateral processes well developed, tapers and slopes down anterolaterally, covers anterior most portion of orbital section. Prethmoid closely attached with anterior most tips of vomer. Nasals are in the form of small bony plate, anterior down to the lateral ethmoid. Frontals large and contributes as the largest anterior portion of neurocranium, with a clear supraorbital lateral-line canal running along its lateral margin. Parietals large, with a sensory canal running on lateroposterior side forming the distal margin of neurocranium. Orbitosphenoid large, distinct, attached dorsally with fontanel and ventrally with dorsovertical flap of parasphenoid. Pterosphenoid pierced, anteriorly attached with distal margin of orbitosphenoid. Parasphenoid long, elongated with dorsovertical flap, attaches anteriorly with vomer and cartilaginous trabecula communis, runs posteriorly till otic region. Prootic with large auditory foramen seems to be the largest bone of the ventral surface of cranium, attached posterolaterally with anterior most part of the subtemporal fossa. Autopterotic forms the posterolateral corner of the neurocranium, ventrally articulates with hyomandibular. On the ventral side of cranium, autopterotic forms the posterolateral wall of the deep subtemporal fossa. Supraoccipital attaches with the parietal on the anterior side, dorsally concave, spine well developed but does not supersedes weberian complex. Exoccipital large, well ossified with little cartilaginous remains, forms posterior wall of the otic capsule and the posterodorsal part of the otic bulla. Basioccipital large, covers ventral portion of the otic bulla, and the articulation between the neurocranium and the first vertebra, it is a posteroventral most point of the occipital region. Basioccipital process well developed, elongated with round concave masticatory plate on the anterior side and it reaches half of weberian complex ventrally.
Hyopalatine arch and opercular series: See Figure 5D. The dorsal edge of the hyomandibular articulates with the autosphenotic, the autopterotic, and the prootic cranium bones and unites with the opercle in anterodorsal portion. Laterally, the hyomandibular overlaps with the posterodorsal margin of the preopercle. Opercle overlaps with interopercle and subopercle anteroventrally. The quadrate well ossified, zone of cartilage, a remnant of the palatoquadrate cartilage is distinctly visible. Its large posteroventral process extends laterally and overlaps the anterior most point of the preopercle. On the anterior side, articular condyle of the quadrate articulates strongly with the anguloarticular. The metapterygoid is well ossified, ventrally attaches with the quadrate on anterior, preopercle and hyomandibular on posterior and overlaps laterally with symplectic. Endopterygoid and ectopterygoid well ossified like metapterygoid and overlaps on dorsal and anterior face of quadrate.
Upper jaw consists of premaxilla and maxilla, relatively large and well ossified. Premaxilla proportionately smaller than maxilla curved anteriorly and free of any joints with maxilla and dentary, while only attached with strong ligaments. Premaxilla articulates with the dentary on posteroventral surface. The lower jaw comprises Meckel’s cartilage, dentary, anguloarticular and the coronomeckelian. Dentary articulates with maxilla on dorsolateral side, broad on posterior edge and pointed towards anterior edge. Coronomeckelian bone presents on dorsal surface of remnant of rod shape Meckel’s cartilage. Kinethmoid a small bone contributing to the ethmoid complex is present in association with maxillae and premaxilla, bound with small ligaments.
Infraorbital series and scleral skeleton: See Figure 5E & F. Five infraorbital bones present, bearing the laterosensory canal which encircles the orbital margin. Infraorbital 1 well developed, wider than infraorbital 2. Infraorbital 2 with laterosensory canal appears like a thin tube, encircles the orbital margin. Infraorbital 3, largest among all and overlaps preopercle. Infraorbital 4 well developed but less wide than infraorbital 1 and 3, infraorbital 5 very small. Supraorbital large and well ossified, covers eye dorsally. Infraorbital 6 absent. Scleral bones well ossified, covers proximal and distal perimeter of eye sclera in lateral view, dorsal and ventral perimeter of eye is cartilaginous (Figure 5E).
Hyoid arch: See Figure 5G & H. Anterior ceratohyal distinct, broad posteriorly, narrows down anteriorly. It is attached anteriorly with dorsal hypohyal and ventral hypohyal and posteriorly with the posterior ceratohyal. Dorsal and ventral hypohyal are small and well ossified with little or no cartilaginous remnants. Large urohyal, with three well spread lateral flanges, attached anteriorly to ventral hypohyal with ligaments. Three well developed thin branchiostegal rays in each half of hyoid arch.
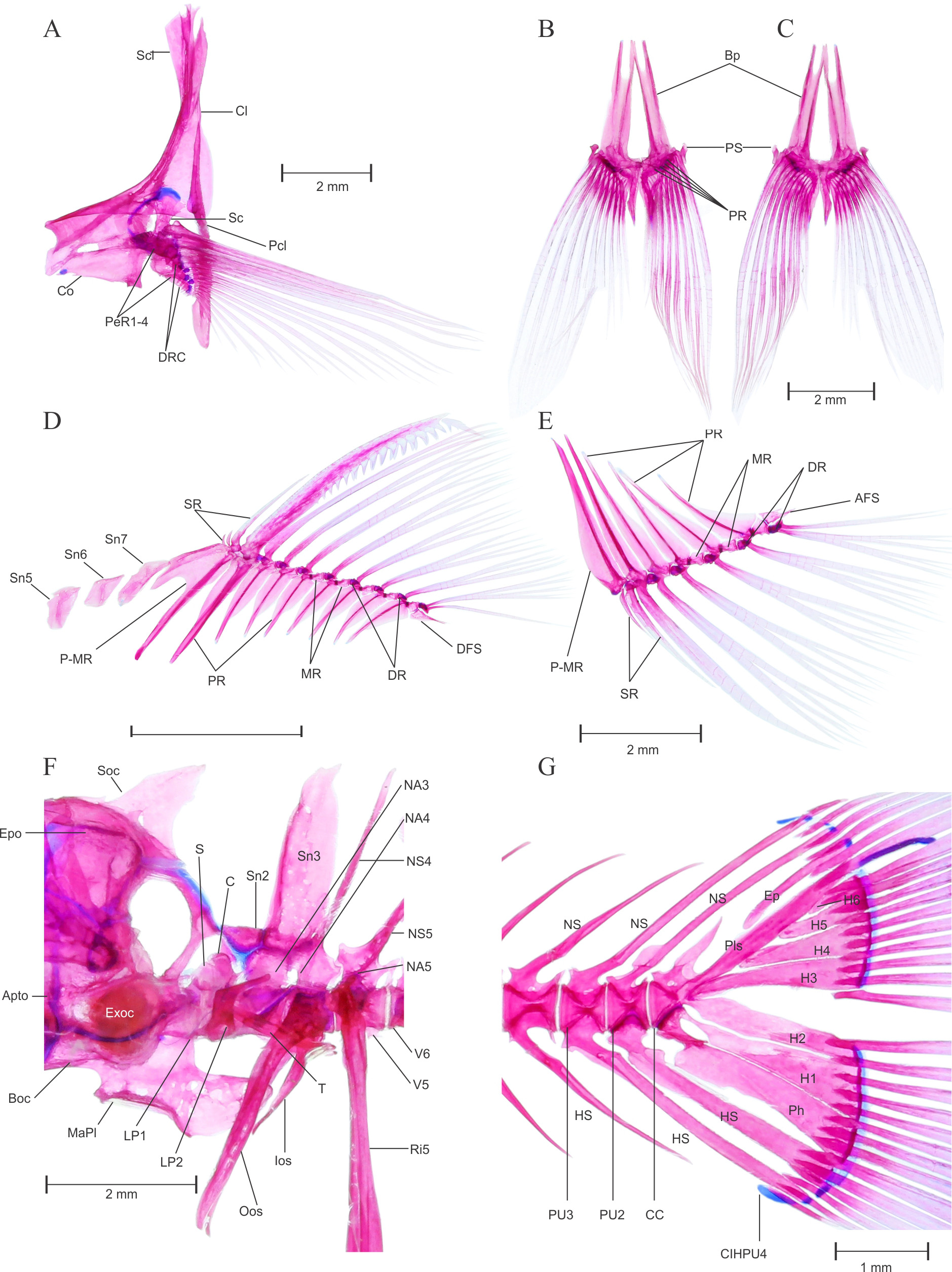
FIGURE 5. Pethia ticto , topotype, male, BNHS FWF 130, 32.2 mm SL; (A) dorsal neurocranium, (B) lateral neurocranium, (C) ventral neurocranium, (D) hyopalatine arch, (E) scleral cartilage and bone; (F) hyoid arch, dorsal view; (G) infraorbital series, lateral view; (H) urohyal bone, (I) Gill arch dorsal view, (J) ventral gill arches dorsal view, (K) dorsal gill arches ventral view and (L-M) 5th ceratobranchial showing pharyngeal teeth in two different angles. Apto, autopterotic; Asph, autosphenotic; Boc, basioccipital; BocPr, basioccipital process; E, ethmoid; Exoc, exoccipital; Epo, epioccipital; Fr, frontal; LE, lateral ethmoid; MaPl, masticatory plate of the basioccipital; Me, mesethmoid; N, nasal; Orsph, orbitosphenoid; Pa, parietal; Pe, prethmoid; Pro, prootic; Psph, parasphenoid; Ptsph, pterosphenoid; Soc, supraoccipital; STF, subtemporal fossa; TrC, trabecula communis; Vo, vomer; Ana, anguloarticular; Apal, autopalatine; Cm, coronomeckelian; De, dentary; Ecpt, ectopterygoid; Enpt, endopterygoid; Hy, hyomandibular; Iop, interopercle; KE, kinethmoid; MC, Meckel’s cartilage; Mpt, metapterygoid; Mx, maxilla; Op, opercle; Pmx, premaxilla; Pop, preopercle; PvPQ, posteroventral process of quadrate; Q, quadrate; Ra, retroarticular; Sop, subopercle; Sy, symplectic; ACh, anterior ceratohyal; BR, branchiostegal ray; DHh, dorsal hypohyal; IO1- 5, infraorbital 1–5; PCh, posterior ceratohyal; Scl B, scleral bone; Scl C, scleral cartilage; So, supraorbital; Uh, urohyal; VHh, ventral hypohyal.
Branchial arches: See Figure 5I –M. Three well ossified cylindrical basibranchial, connected by congregated cartilage; first one is smaller than second and third. Three pairs of small, round, ossified hypobranchials, covered with cartilage, attaches to the respective basibranchial and ceratobranchial. First basibranchial attaches to the basihyal by ligaments; no cartilaginous connection was seen between basihyal and first basibranchial. Fourth and fifth ceratobranchial connects with basibranchial 3 by strong ligaments and basibranchial 4 cartilage remnants. Five pairs of well developed ceratobranchials present. Ceratobranchial 1 with 7 gill rakers on anterior side and 12– 14 on posterior. Ceratobranchial 2 with 14–15 gill rakers along their anterior margins and 15–16 on posterior.
Ceratobranchial 3 with 20 gill rakers on anterior and 18 on posterior margin. Ceratobranchial 4 with 19 gill rakers on anterior and 16 on posterior margin. Ceratobranchial 5 well ossified, toothed having 14 gill rakers on anterior margin. Proximal half of ceratobranchial 5 marked with 3 rows of well ossified conical teeth. Anterior most teeth row comprises 2, middle 3 and posterior most comprises 4 conical and one small tooth (Figure 5L–M). Upper gill arch constitutes by four pairs of well-developed epibranchials. Epibranchial 1 broad and wing shaped followed by relatively less wide epibranchial 2 and 3. Epibranchial 4 narrow bodied. Pharyngobranchial 1 was not located, pharyngobranchial 2 small whereas pharyngobranchial 3 is well developed, pharyngobranchial 4 cartilaginous.
Pectoral Girdle: See Figure 6 View FIGURE 6 A. Pectoral girdle positioned most anteriorly and does not overlap weberian apparatus laterally; articulate with posterolateral surface of neurocranium through vertically aligned supracleithrum and cleithrum. Supracleithrum small, laterally overlaps with cleithrum by articulating more than 3/ 4th area. Cleithrum large, narrow and pointed dorsally, articulates with postcleithrum lateroventrally. Postcleithrum single, elongated and well developed, supports pectoral girdle posteriorly. Coracoid large, rectangular, articulates posteriorly with scapula and pectoral radials, dorsally overlaps with cleithrum. Four large well ossified pectoral radials present. Three distal radials less ossified, with three medial elements predominantly cartilaginous. Eleven distal radial present, first eight distal radial appear to be well ossified whereas remaining three are cartilaginous and abbreviated as distal radial cartilage, supports one unbranched and 12 branched (i. 11.i) pectoral fin rays.
Pelvic girdle: See Figure 6 View FIGURE 6 B–C. Pelvic girdle consists of a pair of thin, flat and elongated basipterygium, each supporting one unbranched and eight branched fin rays (i. 7.i). On the anterior side basipterygium bears two spinelike processes, external and internal. External process slightly elongated than internal. Five well ossified pelvic radials present, pelvic splint is well developed and laterally associated with first unbranched pelvic-fin ray.
Dorsal fin: See Figure 6 View FIGURE 6 D. Dorsal fin constitutes thirteen rays (iv. 8.i) supported by a series of pterygiophores placed between neural spines of vertebrae V8 /V9–V 14/V15 ( Figure 4 View FIGURE 4 A & B). First three pterygiophores constitutes a large proximal–middle radial, articulates with three unbranched supernumerary and one serrated last unbranched dorsal-fin ray, distal radial absent. Posterior to proximal–middle radial first three pterygiophores articulates with respective branched fin rays by having only distal radial, middle radials untraceable in this articulation. Middle radial appears in articulation with distal radial and dorsal base of pterygiophore on fourth proximal radial. All middle and distal radials are well ossified. Last thirteenth fin ray found to be closely united with twelfth branched fin ray, whereas articulated separately with last pterygiophore. 3 (1)–4 (1) free supraneurals, Sn5–8 present on anterior side of dorsal fin.
Anal fin: See Figure 6 View FIGURE 6 E. Anal fin constitutes 9 rays (iii. 5.i) supported by a series of anal pterygiophores placed between hemal spines of vertebrae V17 /V18 (2)– V20 /V21 (1) or V19 /V20 (1) ( Figure 4 View FIGURE 4 A & B). First three pterygiophores constitutes long, elongated proximal–middle radial, articulates with two unbranched supernumerary and last unbranched anal-fin ray, distal radial present with no traces of middle radial. Middle radial appears in articulation with distal radial and dorsal base of pterygiophore on third proximal radial. All middle and distal radials are well ossified. Last sixth branched fin ray found to be closely united with fifth anal-fin ray, whereas articulated separately with last pterygiophore.
Caudal fin: See Figure 6 View FIGURE 6 G. Seven dorsal procurrent caudal-fin rays and six ventral procurrent caudal-fin rays. In cleared and stained specimens, 9 dorsal and 8 ventral principal caudal-fin rays present. Principal caudal-fin rays supported by neural and hemal spines of third preural centrum, epural, pleurostyle, six hypurals and a parhypural. A single inter–hemal spine cartilage of preural centrum 4 present, placed anterior to ventral tip of hemal spine of preural centrum 3 in male. Single epural runs laterally with anterior margin of pleurostyle on dorsal half, distal tip of pleurostyle cartilaginous. Parhypural and hypural 1–6 well developed, broad and ossified. In male, pleural centra 2 supports single neural and hemal spine, pleural centra 3 supports well developed neural and hemal spine which support peduncle arch. Free uroneural absent.
Weberian apparatus: See Figure 6 View FIGURE 6 F. A complex structure made up by first four vertebral centra and their associated elements, covers the anterodorsal portion of swim bladder. The first centrum relatively small but distinct, possesses lateral process 1 (Lp1). Second centra equivalent in size of first, possesses large lateral process of second vertebra (Lp2). Lp2 is more developed and distinct than LP1 and grown laterally in wing shaped processes. Third and fourth centrum is much larger and each equivalent to size of first two. Scaphium and claustrum small, rounded and well ossified, present on dorsal in association with first centra. Tripus well developed, triangular grows posteriorly and overlaps inner arm of the os suspensorium, associated with third vertebral centrum. Outer arms of the os suspensorium much grown, long and reaches behind the postcleithrum bone of pectoral girdle. Neural arch 3 and neural arch 4 well developed and ossified. Supraneural 3 seems to be a second largest bone of weberian apparatus after outer arm of the os suspensorium. Supraneural 2, 3 and neural spine 4 is associated with neural arch 3 and 4. Supraneural 4 absent.
Intermuscular bone: See Figure 4 View FIGURE 4 A–B. Two series of intermuscular bones, epineural and epipleural. Total 24 epineural and 11 epipleural intermuscular bones present; first epineural appears before neural spine of sixth vertebra whereas first epipleural appears before hemal spine of seventieth vertebra. All epipleural and epineural bifurcated at tip and becomes wider in caudal peduncle portion.
Vertebral column: See Figure 4 View FIGURE 4 A–B. 4+4 (2) predorsal vertebrae; 4+26 (2) total vertebrae including posterior most compound centrum, with 4+13 (2) abdominal and 13 (2) caudal vertebrae. Vertebral centra much elongated than deep. Neural pre and postzygopsis appears on all centra posterior to weberian complex. Hemal postzygopsis appear on all caudal vertebrae posterior to 14th (1) or 15th (1) centra. First hemal prezygopsis appears on 21st (1) or 22th (1) centra. Total 11 ribs are associated with 5th to 15th vertebrae.
Coloration in preservative. For general appearance see Figure 1 View FIGURE 1 A; body above lateral scale row grey; head and dorsum dark brown; lower lip, cheek, opercular region below inferior border of eye cream; ventral region uniformly cream white. Body with prominent dark black humeral spot, covering third and fourth lateral line pored scales; a prominent spot on caudal peduncle covering scales 16–19 of lateral scale row, encircling peduncle above. Scale pockets above lateral scale row studded with melanophores. Dorsal fin with 2 longitudinal rows of intense black spots ( Figures 1 View FIGURE 1 , 2 View FIGURE 2 A). Pectoral, pelvic, anal and caudal fins hyaline, without any color bands or spots.
Coloration in life. See Figure 3 View FIGURE 3 A, B. Body yellow. Position of humeral and caudal spot same as described for preserved specimens, spot on caudal peduncle with a golden hoop on its anterior margin. All fins hyaline.
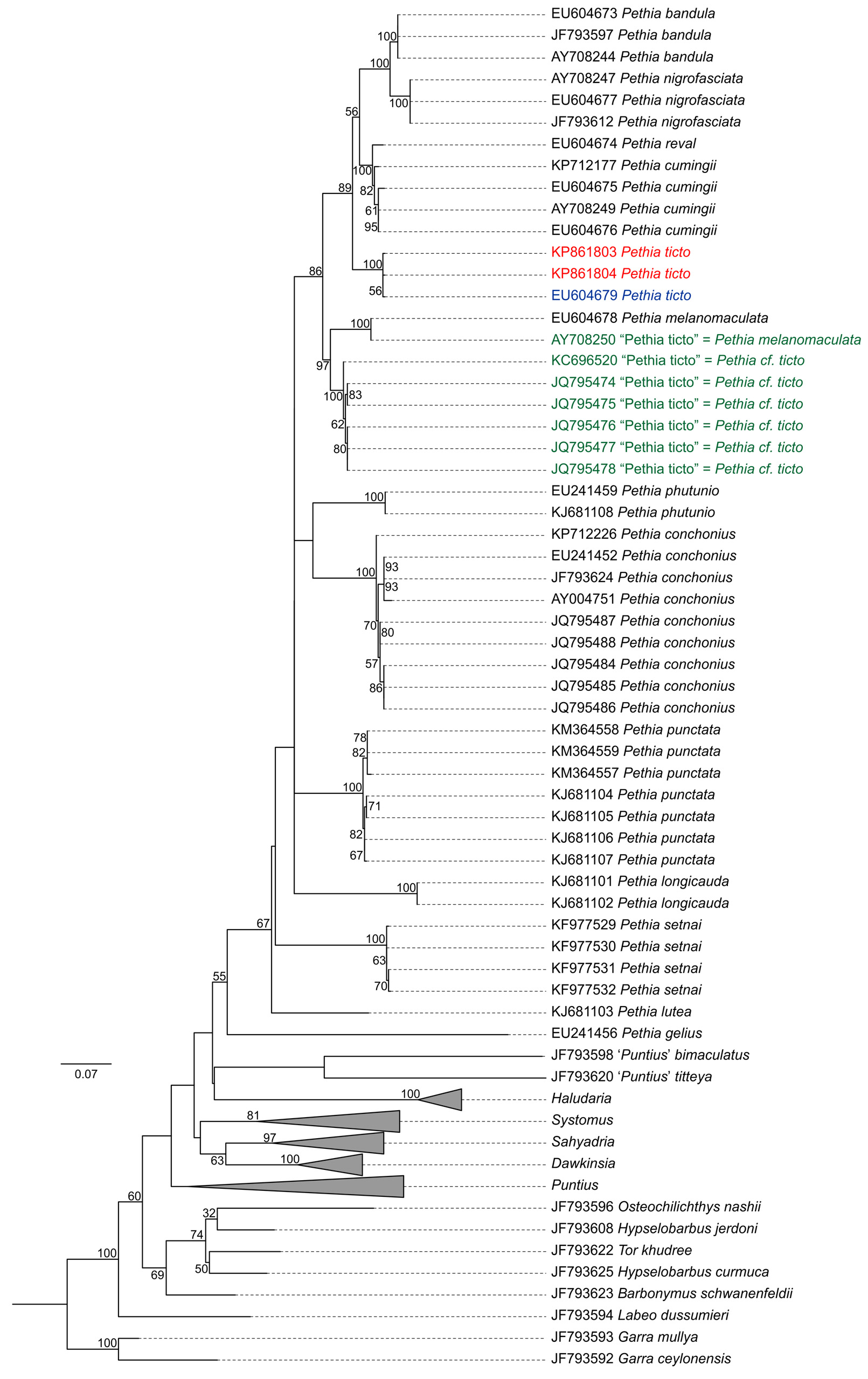
Genetic analysis. Nucleotide substitution rates followed Tamura & Nei (1993) model with gamma distribution and invariant sites (TN93+G+I, BIC = 15828.18, lnL = -6923.37, I = 0.42, G = 0.78). Maximum likelihood tree based on the best fit model is shown in Figure 7 View FIGURE 7 . Based on the comparison of cytb sequences from topotypic material collected in the present study, we suggest that the sequence EU604679 View Materials (from Pethiyagoda et al. 2012) is conspecific to P. t i c t o with a genetic distance of just 0.4%. However, sequence AY708250 View Materials deposited as P. t i c t o is a misidentification with a genetic distance of 10.3% and should be attributed to P. melanomaculata as there is no genetic distance between AY708250 View Materials and P. melanomaculata sequence EU604678 View Materials (from Pethiyagoda et al. 2012). Sequences KC696520 View Materials , JQ795474 View Materials , JQ795475 View Materials , JQ795476 View Materials , JQ795477 View Materials and JQ795478 View Materials deposited as P. ticto are not conspecific to topotypic P. t i c t o and have genetic distance ranging between 8.1–8.4%. We attribute these sequences ( KC696520 View Materials , JQ795474 View Materials , JQ795475 View Materials , JQ795476 View Materials , JQ795477 View Materials and JQ795478 View Materials ) to P. cf. ticto .
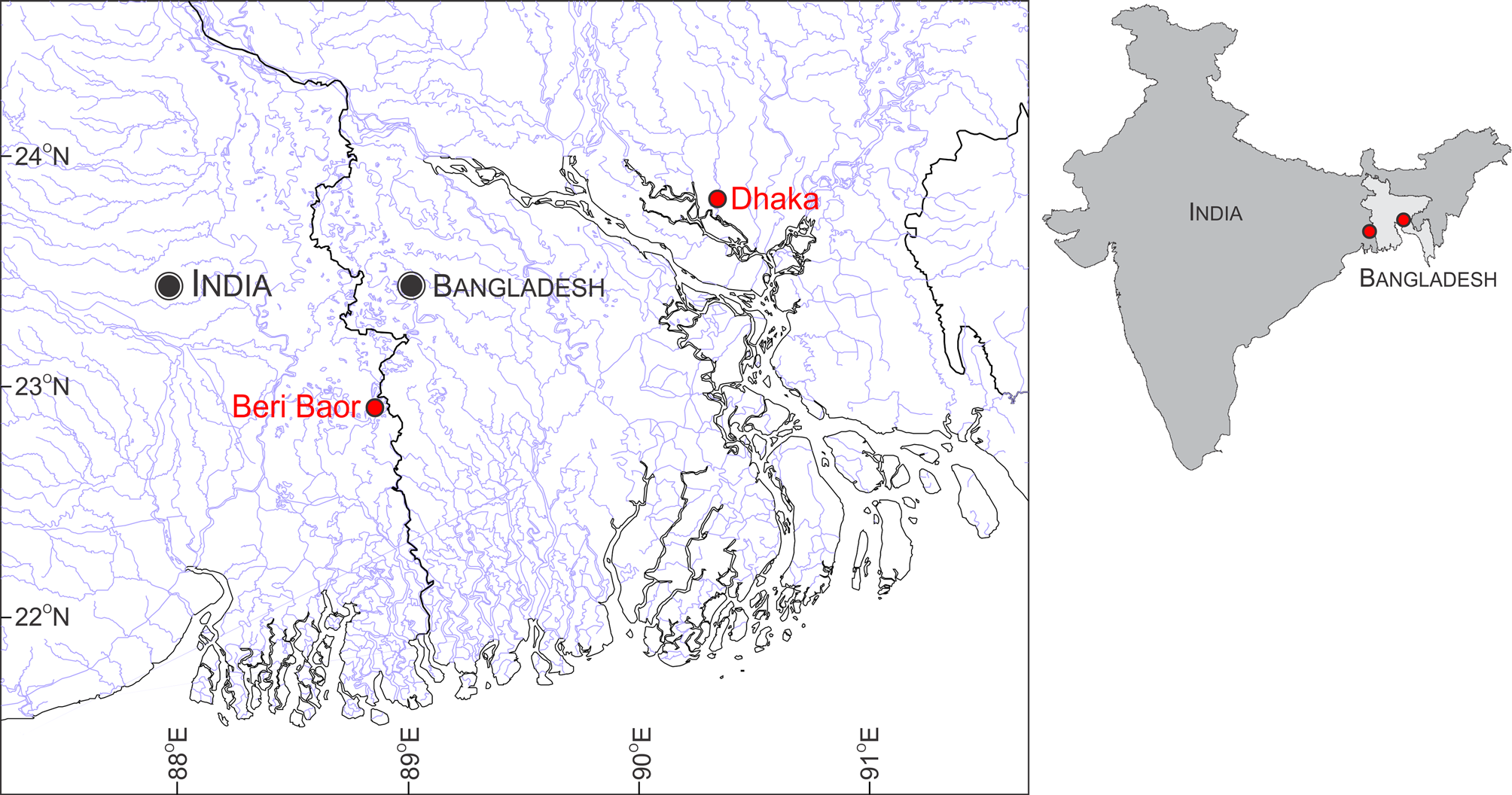
Distribution. In the absence of a precise type locality, we consider the specimens from Beri Baor, Ramnagar, Kolkata, West Bengal, India, as putative topotypes, given that the area is within the general type locality ‘south eastern parts of Bengal’. Although we were not able to procure specimens from Bangladesh for genetic analysis for logistical reasons, the P. t i c t o specimen collected from Dhaka, Bangladesh, was morphologically indistinguishable from the Beri Baor specimens ( Figure 3 View FIGURE 3 ). Specimens identified as P. ticto by Linthoingambi & Vishwanath (2007) from the Brahmaputra River System in Assam and Manipur are morphologically different (see Remarks) and hence may not be conspecific with P. ticto . Further, genetic data of specimens from Lucknow ( JQ795474 View Materials , JQ795475 View Materials , JQ795476 View Materials , JQ795477 View Materials and JQ795478 View Materials ) are also different from P. t i c t o topotypes. Therefore, as currently understood, P. ticto sensu stricto is confined to the Beri Baor, Ramnagar, Kolkata, West Bengal, India, and Dhaka, Bangladesh ( Figure 8 View FIGURE 8 ), both of which lie within the Gangetic floodplain.
Remarks. McClelland (1839) mentioned two names Cyprinus bimaculatus and C. quadrimaculatus , when redescribing Systomus ticto . However, as these name were not accompanied by any descriptive or diagnostic information, Katwate et al. (2014a) considered them to be nomina nuda, not synonyms of P. t i c t o, contrary to Bleeker (1844). Day (1878) considered Systomus tripunctatus Jerdon, 1849 , as a synonym of Barbus ticto . However, the colour pattern of S. tripunctatus provided by Jerdon (1849) does not match with that of P. t i c t o by having three spots over body, two black spots under end of the dorsal and one at the base of the tail vs. two spots over body, one humeral and one on caudal peduncle in P. ticto . Further, S. tripunctatus was described from ‘near the coast in Canara, in Southern India’, and is unlikely to have a range that extends to Bengal. We thus do not consider this species a synonym of P. t icto.
The redescription of P. ticto by Linthoingambi & Vishwanath (2007) was based on specimens from the Brahmaputra River System in Assam and Manipur. Topotypic material from the Ganges basin used in the present study differs from the Brahmaputra form by a lesser number of transverse scale rows ½4/1/3½–4 (vs. ½5/1/5½), lesser body depth 31.5–35.8%SL (vs. 36.8–45.0%SL) and a more compressed body width at dorsal fin origin (body width 10.7–15.4% SL, vs. 16.5–18.4% SL). Further studies are required for testing the conspecificity of these Gangetic and Brahmaputra forms.
Other congeners of Pethia ticto with an incomplete lateral line include P. gelius ( Hamilton, 1822) , P. aurea Knight, 2013 , P. phutunio ( Hamilton, 1822) , P. canius ( Hamilton, 1822) , P. nigripinna (Knight, Rema Devi, Indra & Arunachalam, 2012) , P. macrogramma (Kullander, 2008) , P. reval ( Meegaskumbura, Silva, Maduwage & Pethiyagoda, 2008) , P. erythromycter (Kullander, 2008) , P. cumingii ( Günther, 1868) , P. didi ( Kullander & Fang, 2005) , P. padamya ( Kullander & Britz, 2008) , P. yuensis ( Arunkumar & Tombi Singh, 2003) , P. khugae ( Linthoingambi & Vishwanath, 2007) , P. sharm ai ( Menon & Rema Devi, 1993), P. a t r a ( Linthoingambi & Vishwanath, 2007), P. bandula ( Kottelat & Pethiyagoda, 1991) , P. conchonius ( Hamilton, 1822) , P. nankyweensis (Kullander, 2008) , P. ornatus ( Vishwanath & Laisram, 2004) , P. pookodensis ( Mercy & Jacob, 2007) , P. s ha l y n i u s ( Yazdani & Talukdar, 1975), P. t he l ys (Kullander, 2008), P. manipurensis (Menon, Rema Devi & Viswanath, 2000) , P. longicauda Katwate, Paingankar, Raghavan, Dahanukar, 2014 , P. meingangbii ( Arunkumar & Tombi Singh, 2003) and P. melanomaculata ( Deraniyagala, 1956) . Pethia ticto differs from these by having 6–12 lateral-line pored scales (vs. 3–4 in P. g e l i us, P. aurea and P. phutunio ; 3–5 in P. canius and P. nigripinna ; and 14–23 in P. macrogramma ), 23–26 scales in lateral-scale row (vs. 18–21 in P. reval ; 18–20 in P. erythromycter ; 19–21 in P. cumingii , P. didi and P. padamya ; 21–22 in P. yuensis ; 28–30 in P. khugae and 42 in P. sharmai ), two bands of dark spots on dorsal fin (vs. no bands in P. at r a, P. bandula , P. conchonius , P. nankyweensis , P. ornatus , P. nigripinna , P. pookodensis and P. s h al y ni us; and one band in P. thelys ), ½4 transverse series scales between dorsal fin origin and lateral line (vs. ½ 3 in P. manipurensis and P. longicauda and ½ 5 in P. meingangbii ), dorsal fin with 2 oblique rows of black spots (vs. dorsal fin without any distinct marks in P. melanomaculata ; Batuwita et al. 2015).
Pethia ticto differs from P. punctata in having an incomplete lateral line with 6–12 pored scales (vs. complete lateral line with 23–25 pored scales), 9 predorsal scales (vs. 8), dorsal fin originates behind pelvic fin origin (vs. dorsal fin originates opposite to or slightly in advance of pelvic fin origin) and dark black humeral spot that covers third and fourth lateral line pored scales (vs. humeral spot covers anterior half of forth scale of the row below the lateral line row). Further, the genetic distance in cytb sequences among topotypes of both the species falls between 13.1–13.5%.
While describing Pethia longicauda, Katwate et al. (2014b) attributed sequences JQ795476 View Materials and JQ795475 View Materials to topotypic P. t i c t o. However, this was in error as the voucher specimens used for sequences JQ795476 View Materials and JQ795475 View Materials came from the River Ganges at Lucknow (26º51' N, 80º57' E). Nevertheless, P. longicauda has a 17.2% difference in the cytb gene sequence compared to P. t icto. Pethia lutea Katwate, Katwate, Raghavan, Paingankar & Dahanukar, 2014 , another species recently described from the P. t i c t o complex, differs by a genetic distance of 18.4% in the cytb gene.
TABLE 1. Morphometric data of topotypic Pethia ticto (n = 13, BNHS FWF 127 to 131, WILD- 15 - PIS- 145 to 148, ZSI- WRC P 4360 and DABFUK / FI / 223.
| Character | Mean (sd) | Range |
|---|---|---|
| Morphometrics | ||
| Total length (mm) | 37.1 (4.4) | 32.0–47.2 |
| Standard length (mm, SL) | 28.6 (3.0) | 25.4–36.0 |
| % SL | ||
| Head length (HL) | 30.4 (1.7) | 27.6–33.7 |
| Body depth | 33.6 (1.3) | 31.5–35.8 |
| Body width at Dorsal fin origin | 12.9 (1.4) | 10.7–15.4 |
| Body width at anal fin origin | 8.8 (1.9) | 5.3–11.1 |
| Predorsal distance | 49.6 (1.3) | 47.2–51.7 |
| Dorsal to hypural distance | 53.8 (1.4) | 52.1–57.3 |
| Prepelvic distance | 48.5 (1.8) | 45.5–51.6 |
| Preanal distance | 68.9 (1.2) | 67.6–71.5 |
| Prepectoral distance | 29.9 (1.8) | 27.7–33.3 |
| Dorsal fin length | 29.6 (3.3) | 24.3–34.7 |
| Dorsal fin spine length | 22.1 (3.6) | 16.6–29.6 |
| Length of Dorsal fin base | 18.7 (1.9) | 15.4–22.1 |
| Pectoral–fin length | 22.7 (2.2) | 19.3–26.3 |
| Anal–fin depth | 19.1 (2.1) | 14.3–21.7 |
| Caudal–peduncle length | 20.4 (1.3) | 17.0–22.2 |
| Caudal–peduncle depth | 14.0 (0.7) | 12.7–15.1 |
| % HL | ||
| Head depth | 77.1 (4.8) | 69.9–87.0 |
| Head width | 49.6 (2.3) | 45.9–54.4 |
| Snout length | 24.5 (1.7) | 22.2–27.6 |
| Eye diameter | 34.3 (2.3) | 30.8–37.1 |
| Inter orbital width | 35.3 (3.3) | 29.5–39.4 |
| Meristics | ||
| Lateral row scales | 23–26 | |
| Number of lateral line pores | 6–12 | |
| Dorsal–fin spine serrae | 11–21 | |
| Transverse scale rows | ½4/1/3½–4 | |
| Predorsal scales | 9 | |
| Prepelvic scales | 10–12 | |
| Preanal scales | 16–19 | |
| Circumpeduncular scales | 12 | |
| Dorsal–fin rays | iii8 | |
| Pectoral–fin rays | i12–15 | |
| Pelvic–fin rays | i7 | |
| Anal–fin rays | iii5 | |
| Caudal–fin rays (procurrent) | 4–6+4–6 | |
| Caudal–fin rays (principal) | 8–9+8–9 |
| BNHS |
Bombay Natural History Society |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Pethia ticto ( Hamilton, 1822 )
| Katwate, Unmesh, Raghavan, Rajeev & Dahanukar, Neelesh 2015 |
Systomus ticto (
| Pethiyagoda 2012: 81 |
| Menon 1999: 103 |
| Motwani 1957: 11 |
| Menon 1952: 268 |
| Hora 1939: 263 |
| Day 1878: 576 |
| Day 1871: 290 |
| Gunther 1868: 153 |
| Bleeker 1844: 260 |
Cyprinus ticto
| Hamilton 1822: 314 |