Abstract
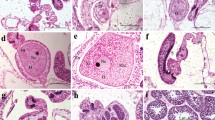
Sinularia flexibilis Quoy and Gaimard, 1833 is a dominant soft coral on many Indo-Pacific coral reefs, and has been found to release toxic compounds (diterpenes), which cause tissue necrosis and death in nearby scleractinian corals. This study investigates how S. flexibilis-derived diterpenes inhibit the development of Acropora tenuis Dana, 1846 and Montipora digitata Dana, 1846 eggs and larvae in vitro. Collection and experimental sites at Magnetic (146°49'E; 19°8'S) and Orpheus (146°28'E; 18°32'S) Islands, Queensland, Australia, were utilized during the spawning seasons of 1989–1992. Freshly spawned coral eggs were placed in solutions of three different terpenes, flexibilide, dihydroflexibilide and sinulariolide, at 5 and 10 ppm, before, during, and after fertilisation. The majority of eggs which were fertilised in the presence of the diterpenes lost their cellular integrity and burst just a few hours after treatment. Terpenes were not toxic to unfertilised eggs, nor to 24 h-old embryos, although sperm ceased swimming activity after 1 h of treatment. The terpenes were not fatal to the sperm because fertilisation still occurred in their presence. The ability of the soft coral-derived diterpenes to inhibit cell division suggests that they may have potential applications in cancer chemotherapy.
Similar content being viewed by others
References
Aceret TL (1994) Screening of biomedical substances derived from marine organisms. Ph. D. dissertation. James Cook University of North Queensland, Townsville, Australia
Aliño PM, Coll JC (1989) Observations of the synchronized mass spawning and post settlement activity in octocorals in the Great Barrier Reef, Australia: biological aspects. bull mar Sci 45:697–707
Arey LB (1974) Developmental anatomy. 7th edn. W. B. Saunders, Philadelphia
Atoda K (1951) The larva and postlarval development of some reef buiding corals. III. Acropora breuggenni (Brook). J Morph 89:1–13
Babcock RC, Bull GD, Harrison PL, Heyward A, Oliver JK, Wallace CC, Willis BL (1986) Synchronous spawnings of 105 seleractinian coral species on the Great Barrier Reef. Mar Biol 90:379–394
Babcock RC, Heyward AJ (1986) Larval development of certain gamete-spawning scleractinian corals. Coral Reefs 5:111–116
Chapman DM (1974) Cnidarian histology. In: Muscatine L, Lenhoff HM (eds) Coelenterate biology — reviews and perspectives. Academic Press, New York, pp 2–92
Coll JC, Bowden BF (1986) The application of vacuum liquid chromatography to the separation of terpene mixtures. J nat Products (Lloydia) 49:934–936
Coll JC, Bowden BF, Meehan GV, Konig GM, Carroll AR, Tapiolas DM, Aliño PM, Heaton A, De Nys R, Leone PA, Maida M, Aceret TL, Willis RH, Babcock RC, Willis BL, Florian Z, Clayton MN, Miller RL (1994) Chemical aspects of mass spawning in corals. I. Sperm-attractant molecules in the eggs of the scleractinian coral Montipora digitata. Mar Biol 118:177–182
Coll JC, Bowden BF, Sammarco PW, Williams WT, Bakus GJ (1982a). Chemical defenses in soft corals (Coelenterata: Octocorallia) of the Great Barrier Reef: a study of comparative toxicities. Mar Ecol Prog Ser 8:271–278
Coll JC, Bowden BF, Tapiolas DM, Dunlap WC (1982b). In situ isolation of allelochemicals released from soft corals (Coelenterata: Octocorallia): a totally submersible apparatus. J exp mar Biol Ecol 60:293–299
Coll JC, Leone PA, Bowden BF, Carroll AR, König GM, Heaton A, de Nys R, Maida M, Aliño PM, Willis RH, Babcock RC, Florian Z, Clayton MN, Miller RL, Alderslade PN (1995) Chemical aspects of mass spawning in corals. II. (-)-Epi-thunbergol, the sperm attractant in the eggs of the soft coral Lobophytum crassum (Coelenterata, Octocorallia) Mar Biol (in press)
Coll JC, Sammarco PW (1983) Terpenoid toxins of soft corals (Cnidaria. Octocorallia) their nature, toxicity and ecological significance. Toxicon (Suppl), 3:69–72
Faulkner DJ (1986) Marine natural products. Nat Product Rep 3:1–33
Faulkner DJ (1990) Marine natural products. Nat Product Rep 7: 269–309
Faulkner DJ (1991) Marine natural products. Nat Product Rep 8: 97–147
Fusetani N (1987) Marine metabolites which inhibit development of echinoderm embryos. In: Scheuer PJ (ed) Bioorganic marine chemistry. 1. Springer-Verlag, New York, pp 61–92
Giese AC (1968) Cell physiology. 3rd edn. W. B. Saunders Co., Philadelphia
Gumbiner B, Stevenson B, Grimaldi A (1988) The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J Cell Biol 107:1575–1587
Harrison PL, Babcock RC, Bull GD, Oliver JK, Wallace CC, Willis BL (1984) Mass spawning in tropical reef corals. Science, NY 223:1186–1189
Harrison P, Wallace CC (1990) Reproduction, dispersal and recruitment of scleractinian corals. In: Dubinsky Z (ed) Coral reefs. Elsevier, Amsterdam, pp 133–207
Hart NN, Becker KA, Wolenski JS (1992) The sperm entry site during fertilisation of the zebrafish. Molec Reprod Dev 32:217–228
Heyward AJ, Babcock RC (1986) Self-and cross-fertilization in scleractinian corals. Mar Biol 90:191–195
Heyward AJ, Collins JD (1985) Growth and sexual reproduction in the scleractinian coral Montipora digitata Dana. Aust J mar. Freshwat Res 36:441–446
Kazlauskas R, Murphy PT, Wells RJ, Schonlholzer P, Coll JC (1978) Cembranoid constituents from an Australian collection of the soft coral Sinularia flexibilis. Aust J Chem 31:1817–1824
La Barre SC, Coll JC, Sammarco PW (1986) Defensive strategies of soft corals (Coelenterata: Octocorallia) of the Great Barrier Reef. II. Therelationship between toxicity and feeding deterrence. Biol Bull mar biol Lab, Woods Hole 171:565–576
Maida M (1993) Allelopathic effects of alcyonacean soft corals on the settlement and early development of scleractinian corals. Ph.D. dissertation. James Cook University of North Queensland, Townsville, Australia
Munro MGH, Luibrand RT, Blunt JW (1987) The search for antiviral and anticancer compounds from marine organisms. In: PJ Scheuer (ed) Bioorganic marine chemistry. 1. Springer-Verlag, New York, pp 96–176
Onta T (1991) Initial stages of sperm-egg fusion in the freshwater teleost Rhodeus ocellatus ocellatus. Anat Rec 229:195–202
Oppenheimer SB (1985) Cancer: a biological and clinical introduction. 2nd edn. Jones & Bartlett Publishers, Inc., Boston
Ruggieri GD, Baslow MH, Jakowska S, Nigrelli RF (1961) Developmental aberrations in sea urchin eggs induced by sponge extracts. Am Zool 1:384–385
Sammarco PW, Coll JC, La Barre SC, Willis B (1983) Competitive strategies of soft corals (Coelenterata: Octocorallia): allelopathic effects on selected scleractinian corals. Coral Reefs 1:173–178
Sokal RR, Rohlf FJ (1981) Biometry. The principles and pratice of statistics in biological research 2nd edn. WH Freeman & Co., New York
Taggart DA, O'Brien HP, Moore HDM (1993) Ultrastructural characteristics of in vivo and in vitro fertilisation in the grey shorttailed oppossum Monodelphis domestica. Anat Rec 237:21–37
Takeichi M (1988) The cadherins: cell-cell adhesion molecules controlling animal morphogenesis. Development (UK) 102:639–655
Terasaki M, Jaffe LA (1991) Organisation of the sea urchin egg endoplasmic reticulum and its reorganisation at fertilisation. J Cell Biol 114:929–940
Tursch B, Breakman JC, Daloze D, Kaisin M (1978) Terpenoids from coelenterates. In: Scheuer PJ (ed) Marine natural products: chemical and biological perspectives. 2. Academic Press, New York, pp 247–296
Webb L, Coll JC (1983) Effects of alcyonarian coral terpenes on scleractinian coral photosynthesis and respiration. Toxicon (Suppl) 3:485–488
Willis BL, Babcock RC, Harrison PL, Oliver JK, Wallace CC (1985) Patterns in the mass spawning of corals on the Great Barrier Reef from 1981 to 1984. Proc. 5th int coral Reef Congr 4:343–348 [Gabrié C et al. (eds) Antenne Museum-EPHE, Moorea, French Polynesia]
Author information
Authors and Affiliations
Additional information
Communicated by G.F. Humphrey, Sydney
Rights and permissions
About this article
Cite this article
Aceret, T.L., Sammarco, P.W. & Coll, J.C. Effects of diterpenes derived from the soft coral Sinularia flexibilis on the eggs, sperm and embryos of the scleractinian corals Montipora digitata and Acropora tenuis . Marine Biology 122, 317–323 (1995). https://doi.org/10.1007/BF00348945
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00348945