Abstract
The introduction of freshwater fish species is a leading cause of aquatic biodiversity erosion and can spread parasites to native populations. Hidden diversity evidenced by recent taxonomic revisions can add further complexity to the issue by rendering biological assessment data incomplete. The Eurasian minnows Phoxinus are one such example of cryptic diversity, with several described species being invasive. Current non-native fish populations in the small Mediterranean island of Corsica (France) are the result of successive waves of introductions, including several Phoxinus species. This study aims at determining which Phoxinus species were introduced to Corsica using the cytochrome oxidase subunit I barcoding marker, reconstructing their introduction routes and examining their parasite communities. The study found four species in Corsica: Phoxinus phoxinus and Phoxinus csikii mainly in the northernmost studied drainage basin and Phoxinus dragarum and Phoxinus septimaniae in the Tavignano drainage basin. P. phoxinus and P. csikii were most likely introduced through a live bait wholesaler while P. dragarum and P. septimaniae were probably introduced by recreational anglers bringing their bait from continental France. The molecular study of their Gyrodactylus (Platyhelminthes: Monogenea) parasites with the ITS marker allowed us to hypothesize inter-drainage basin secondary introduction routes for P. phoxinus and P. dragarum. In several sampling sites, Phoxinus minnows had black spot disease caused by encysted metacercariae of Digenea, likely Posthodiplostomum cuticola. These parasites were also found on the brown trout Salmo trutta in a locality where this patrimonial species co-occurs with Phoxinus minnows. Barcoding should be used in fish communities monitoring to help to accurately identify cryptic species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The introduction of non-native species, especially fish, is one of the leading causes of the decline of fauna in freshwater ecosystems at global scale, impacting both the abundance and distribution of native species (Cambray 2003; Clavero and Garcia-Berthou 2005; Milardi et al. 2018). Fish introductions have diverse impacts on both invaded ecosystems and native species, ranging from alteration of interactions between fish (e.g. increased predation pressure), increase in prey availability for native predators or competition for trophic resources, alteration of trophic web structure possibly leading to habitat alteration (e.g. eutrophication), and genetic impacts through hybridization and introgression (Ribeiro and Leunda 2012; Witkowski and Grabowska 2012; Ellender and Weyl 2014; Tadese and Wubie 2021). An additional, particularly noteworthy consequence of species introduction is the co-introduction of associated parasites and/or pathogens (Taraschewski 2006; Ribeiro and Leunda 2012; Ellender and Weyl 2014; Goedknegt et al. 2016; Tadese and Wubie 2021). Such organisms are frequently introduced along with their hosts in a new area (Lambert 1997; Prenter et al. 2004; Taraschewski 2006). One reason to focus on these parasite co-introductions is that this phenomenon may have a serious impact as the lack of co-evolution between non-native parasites and native hosts can result in the lack of an adequate immune response to the infection. The scale of the problem may be underestimated when biodiversity assessment data are either lacking or incomplete, which can be the case when dealing with cryptic species. Cryptic species are two or more species usually reported as a single one because of their indistinguishable morphology and a lack of systematic studies (Bickford et al. 2007). One such example of cryptic diversity is the case of minnows Phoxinus spp. (Leuciscidae) (Kottelat 2007; Palandačić et al. 2017; Corral-Lou et al. 2019; Denys et al. 2020), small freshwater fish widely distributed across Eurasia, for which reliable diagnosis on the field is impeded by the difficulty of observing the diagnostic characters (Bianco 2014), except the nuptial coloration pattern shown in French species (Denys et al. 2020), i.e. only during their spawning period. There are currently 26 valid Phoxinus species in Eurasia (Berg 1949; Mitrofanov et al. 1987; Chen 1988; Kottelat 2006, 2007; Bianco and De Bonis 2015; Zhang and Zhao 2016; Palandačić et al. 2017; Bogutskaya et al. 2020, 2023; Denys et al. 2020; Dyldin et al. 2023; Turan et al. 2023; Artaev et al. 2024; Bayçelebi et al. 2024), 5 more molecular lineages potentially corresponding to distinct species (Palandačić et al. 2017) and the taxonomy of this genus is still under study, owing notably on the emergence of molecular tools. Within the genus, several introduction events have been documented e.g. Phoxinus csikii Hankó, 1922 and Phoxinus septimaniae Kottelat 2007 were likely introduced in the lower and middle Rhine catchment systems (Netherland, Belgium and Germany) (Palandačić et al. 2020, 2022); the latter species was also introduced in the western Po river basin (Italy), possibly during the same period with the growing popularity of trout angling (De Santis et al. 2021). Corral-Lou et al. (2019) highlighted the introduction in Catalonia of P. septimaniae and of a lineage from the Garonne which may be Phoxinus dragarum Denys, Dettai, Persat, Daszkiewicz, Hautecoeur and Keith, 2020 which is endemic to the Garonne drainage basin. Similarly, Denys et al. (2020) affirmed the introduction of P. dragarum in the Guadalquivir drainage basin on the basis of the nuptial coloration of the specimen illustrated by Sáez-Gómez and Prenda (2019). Garcia-Raventós et al. (2020) noted the introduction of a population from the Charente drainage basin (Western France) in the Sousa river (Portugal). Introductions of Phoxinus were due to their use as live bait for angling, or via contamination of Salmonidae used to reinforce stocks to enhance angling (Museth et al. 2007; Miró and Ventura 2015; Garcia-Raventós et al. 2020). These introduction events contributed to the alteration of the distribution of Phoxinus minnows and have made their management more complex. Knowledge of this genus’ taxonomy has evolved over the last fifteen years (Kottelat 2007; Palandačić et al. 2017, 2020, 2022; Corral-Lou et al. 2019; Denys et al. 2020; Garcia-Raventós et al. 2020; De Santis et al. 2021).
The small Mediterranean island of Corsica (France) displays a unique freshwater fish stock, with a native fish fauna composed of only 4 native fish species: the European eel Anguilla anguilla (Linnaeus 1758), the brown trout Salmo trutta Linnaeus, 1758, the freshwater blenny Salariopsis fluviatilis (Asso 1801) and the three-spined stickleback Gasterosteus aculeatus Linnaeus, 1758; and naturally devoid of several Cypriniformes occurring in the Ibero-Franco-Italian region (Roule 1933; Changeux 1998); and more than 20 non-native species resulting from successive waves of introductions into the island's rivers and lakes (Roche and Mattei 1997; Roché 2001). These introductions started at the end of the nineteenth century with the addition of the mosquitofish Gambusia holbrooki Girard 1859 in an attempt at biological control of malaria vectors (mosquito control). The introductions then continued in the 1970s with the release of the brook trout Salvelinus fontinalis (Mitchill 1814) and the domestic brown trout Salmo trutta Linnaeus, 1758 in mountain lakes. Several species such as the roach Rutilus rutilus (Linnaeus, 1758), the rudd Scardinius erythrophthalmus (Linnaeus, 1758), the tench Tinca tinca (Linnaeus, 1758), the carp Cyprinus carpio Linnaeus, 1758 and the pikeperch Sander lucioperca (Linnaeus, 1758) were then introduced into artificial lakes and probably dispersed by anglers to rivers. Several non-controlled introductions occurred afterwards at unknown dates, such as those of Carassius sp., the common perch Perca fluviatilis Linnaeus, 1758 and gudgeons Gobio spp. Being an insular environment, Corsica is especially sensitive to species introductions (Towns et al. 2006; Donlan and Wilcox 2008). The arrival of Phoxinus minnows in Corsica are one such example of fish introductions that may have an impact on native fish populations e.g. the heritage species Salmo trutta Linnaeus, 1758. According to Denys et al. (2020), two species of Phoxinus are known to occur on the island: Phoxinus phoxinus Linnaeus, 1758 in the Golo river and P. dragarum in the Tavignano river. However, this data was acquired at only two localities on the island, and owing to the difficulty of discriminating between Phoxinus species outside their spawning period, the specific diversity of this genus has yet to be explored in Corsica.
Parasites have been used as a tool for assessment of conservation issues, helping to untangle the introduction history of their hosts, to identify their origin and spreading routes by acting as proxies for their hosts’ genealogy and ecology (Whiteman and Parker 2005; Nieberding and Olivieri 2007; Gagne et al. 2022). The shorter generation time and smaller population size of parasites, compared to their hosts, allow the use of parasites as markers to clarify the origin and dispersal of invasive species (Nieberding and Olivieri 2007; Gagne et al. 2022). For this purpose, considerable attention has been paid to viruses (e.g. Biek et al. 2006; Allen et al. 2010; Wilfert and Jiggins 2014). However, macroparasites have also been used successfully to gain insight into the origin of introduced hosts, their introduction routes and vectors, to highlight contemporary and historical contacts between host populations, to identify the source population of migratory individuals, past migrations and differentiation events (Wickström et al. 2003; Nieberding et al. 2004, 2006; Criscione et al. 2006; Reshetnikov et al. 2011; Huyse et al. 2015; Kmentová et al. 2019; Šimková et al. 2022). A key feature with regard to the ability of a parasite to be a useful marker is its shared history with its host, which is dependent on the strength of the host-parasite interaction and thus on its host specificity, on the absence or presence of intermediate hosts and on the absence or presence of a free-living stage (Page 2003; Clayton and Johnson 2003; Charleston and Perkins 2006; Nieberding and Olivieri 2007). Monogeneans, with their often strong association with their host, their direct life cycle and direct transmission, are good candidates to study their fish host's introductions, dispersion, biogeography and evolutionary history (Pariselle et al. 2011; Lumme et al. 2016; Kmentová et al. 2019; Benovics et al. 2020; Šimková et al. 2022; Rahmouni et al. 2023a).
The aims of this study were (1) to inventory the Phoxinus species introduced to the small Mediterranean island of Corsica and their intraspecific variability, and to retrace their introduction routes through haplotype networks and parasite fauna, (2) to study their parasite communities for the first time in this region and (3) assess their potential impact on the native fish fauna, especially native S. trutta.
Material and methods
Study area and sample collection
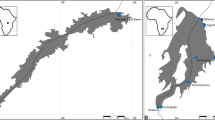
A total of 225 Phoxinus minnows were taken from 12 different freshwater sampling localities across the Haute-Corse Département (Corsica, French Mediterranean, Fig. 1, Table 1). The sampling localities cover a total of 10 rivers and five drainages basins. The samplings were conducted from June to October 2022 by electrofishing in compliance with French legislation and with the help of the French Agency of Biodiversity (OFB) as well as the Angling Federation of Corsica (FDAAPPMA2). Sampled watercourses were selected to include all rivers for which Phoxinus minnow abundance was sufficient, on the basis of data obtained through the European Union Water Framework Directive fish monitoring conducted by the OFB.
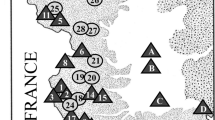
Sampling localities for Phoxinus minnows in Corsica, with the corresponding proportions of Phoxinus species and corresponding Gyrodactylus clusters. Locality number (river, locality name): 1 (Asco, Moltifao); 2 (Casaluna, Gavignano); 3 (Tartagine, Castifao); 4 (Golo, Barchetta); 5 (Fium’Alto, Taglio-Isolaccio); 6 (Vecchio, Venaco); 7 (Tavignano, Altiani); 8 (Tavignano, Piedicorte-di-Gaggio); 9 (Tavignano, Saint-Georges); 10 (Corsigliese, Pancheraccia); 11 (Fium’Orbo, Ghisonaccia); 12 (Abatesco, Prunelli-di-Fium’Orbo). Diamonds are the major cities in Corsica, n is the number of individuals of the corresponding species in the corresponding locality
Dissection and parasitological examination
Phoxinus minnows were euthanized in compliance with French legislation (NOR: AGRG1238753A), transported on ice in individual bags to the laboratory, and kept on ice until examination. Each individual was weighed to the nearest 0.1 g (TW, in g), and measured to nearest millimeter (TL, in mm). Fin-clips were preserved in 95% ethanol for molecular studies. The stomach, intestine, swim bladder, spleen, and liver were placed in Petri dishes with physiological saline and examined under a stereomicroscope for parasites. The skin, gills, fins, mouth, and abdominal cavity were also checked for parasites. Brains were checked for Diplostomum phoxini (Faust, 1959) metacercariae by squashing the brain gently between two microscope slides as described in Müller (1995). All parasites collected were preserved in 70% or 90% ethanol. A subsample of Gyrodactylus (Platyhelminthes: Monogenea) were used for further molecular analysis.
Statistical analysis
Parasite indices were calculated following the terminology of Bush et al. (1997): prevalence is the number of hosts infected with at least one individual of a particular parasite species divided by the number of hosts examined and expressed as a percentage; mean abundance is the total number of individuals of a given parasite species in a sample divided by the total number of hosts in that sample; and mean intensity is the total number of individuals of a given parasite species in a sample divided by the number of infected hosts in that sample. As species are considered bio-indicators when their abundance and/or frequency in a particular habitat are significantly higher in this habitat (Mouillot et al. 2002), an analysis of indicator values (IndVal) (Dufrêne and Legendre 1997) was used to combine the parasite species’ relative abundance (specificity) and relative frequency (fidelity) for a given variable. Specificity is the mean abundance of a parasite species in a given group of Phoxinus minnows divided by the same parasite abundance infecting all Phoxinus minnows. Fidelity is defined as the percentage of Phoxinus minnows in a given group infected by a given parasite species. The IndVal analysis’s capacity to include both specificity and fidelity in the same index constitutes an advantage over classical statistical tests (e.g., ANOVA) when looking for indicator species in highly variable communities, such as parasites (Mouillot et al. 2002). Calculations of IndVal and associated p values (10,000 permutations) were conducted using the labdsv R package (Roberts 2019).
Molecular analyses
For minnows, a DNA barcoding approach sensu (Hebert et al. 2003) was done with the cytochrome oxidase subunit 1 (COI) marker. DNA extraction, PCR, sequencing and sequences cleaning follow Denys et al. (2020). Concerning parasites, total DNA was extracted following the same protocol as for minnows.
Concerning Gyrodactylus parasites, total DNA was extracted with a QIAamp ® DNA Micro kit (QIAGEN) following the manufacturer’s instructions. The D1 + D2 regions of the 28S rDNA gene was amplified using the forward primer C1 5’-ACCCGCTGAATTTAAGCAT-3’ and the reverse primer D2 5’-TGGTCCGTGTTTCAAGAC-3’ (Wu et al. 2005) and a partial fragment of the Internal Transcribed Spacer (ITS2) region was amplified using the forward primer ITS4.5 5’-CATCGGTCTCTCGAACG-3’ and the reverse primer IST2 5’-TCCTCCGCTTAGTGATA-3’ (Matejusová et al. 2001). DNA was amplified by PCR in a final 20 µL volume containing 1 µL DMSO, 1 µL of dNTP 6.6 µmol/L, 0.15 µL of Qiagen Taq DNA polymerase, using 2 µL of the buffer provided by the manufacturer and 0.4 µL of each primer at 10 pmol/L; 3 µL of DNA extract was added. After 3 min denaturation at 95 °C (hot start), the PCR was run for 50 cycles (30 s at 94 °C; 1 min at 50 °C for ITS2 and 56 °C for 28S; 1 min 30 s at 72 °C), with a 3 min terminal elongation at 72 °C on a Bio-Rad t100™ thermal cycler. Successful PCRs were selected on ethidium-bromide stained agarose gel. Sanger sequencing was performed in both directions by a commercial company (Eurofins) (http://www.eurofins.fr).
Species identification, phylogenetic grouping and haplotype networks
Phoxinus COI sequences were compared with a molecular dataframe from different publications (Geiger et al. 2014; Knebelsberger et al. 2015; Behrens-Chapuis et al. 2015, 2021; Thalinger et al. 2016; Schönhuth et al. 2018; Denys and Manne 2019; Denys et al. 2020; De Santis et al. 2021; Zangl et al. 2022; Table S1). Aligning, p-distances and NJ-tree reconstruction based on the DNA barcodes were performed under MEGA X (Kumar et al. 2018) with the Kimura 2 parameter model (K2P; Kimura 1980). Bootstrap values (Felsenstein 1985) with 1000 replicates were also calculated for evaluating the robustness of clusters.
Median-joining networks were built from the COI datasets of each species using Network v.4.6 (Bandelt et al. 1999). We applied a maximum parsimony algorithm and the criterion “frequency > 1” to simplify the complex branching scheme and generate networks representing the most parsimonious relationships. Genetic diversity indices (haplotype diversity (Hd), number of polymorphic site (S) and number of haplotypes (h)) were calculated with DnaSP V6 (Rozas et al. 2017).
Gyrodactylus delineation was performed as follows: data processing and sequence assembling were done with Geneious Prime ® 2020.2.4 (http://www.geneious.com). Sequences were aligned with MAFFT alignment (Katoh et al. 2002). PartitionFinder v.2.1.1 (Lanfear et al. 2012) was used to estimate the best evolution model for the Bayesian phylogenetic inference analyses selected under the Bayesian Information Criterion (GTR + G for ITS2 and 28S). The percentage of divergence between sequences was calculated in Geneious Prime. The phylogenetic tree was constructed with MrBayes v.3.2.6 (Ronquist et al. 2012). Two independent analyses were run for 10 million generations, sampling every 200 generations. The convergence of the two analyses was checked and the tree obtained is a consensus with ten percent of the trees discarded as burn-in. Sixteen sequences were obtained for the phylogenetic reconstruction and two sequences of Benedenia armata were added from GenBank (LC602801.1 for ITS2 and LC408961.1 for 28S) as out group. A total of 16 sequences were used for the phylogenetic reconstruction based on ab alignment of 1390 base pairs (pb).
Results
Diversity of introduced Phoxinus in Corsica
The Phoxinus minnows sampled in Corsican rivers were molecularly identified at the species level as P. phoxinus, P. dragarum, P. septimaniae and P. csikii, as supported by the phylogenetic tree (Fig. 1, S1, Table 1, S2,). The distribution of these recorded species seems to be dependent on the drainage basin, with P. csikii recorded exclusively from the Golo drainage basin and P. septimaniae from the Tavignano drainage basin. P. dragarum was recorded only in the Tavignano drainage basin and those to the south of it. P. phoxinus is the majority of the identified individuals in both northernmost sampled drainages basins (Golo and Fium’Alto) (Fig. 1, S1).
Five P. dragarum haplotypes were detected in Corsica (Fig. 2). H7 is shared by 19 individuals sampled in the Fium’Orbo and specimens originating from the Garonne drainage basin in the Pyrenees and the Landes (six sequences), H4 is shared by 72 P. dragarum from the Tavignano and one specimen from the Garonne drainage basin in the Massif Central (Fig. 2a). Three haplotypes (H5, H6 and H11; respectively 12, 15 and 5 individuals) were new and did not correspond to any available sequence. Three haplotypes were detected in Corsica for P. septimaniae (Fig. 2b): no correspondence was found for H4 and H5 (one and five individuals respectively), which are separated from specimens originating from the Rhone drainage basin by two and three mutational steps, respectively. The haplotype H24 recorded from the Tavignano in Corsica (6 sequences) was shared by minnows sampled in several drainage basins: Mediterranean and Rhone (France, 7 sequences), Middle Rhine (Germany, 12 sequences) and Po (Italy, one sequence). Three P. phoxinus haplotypes were recorded in Corsica of which H11 was the major one (69 sequences) and was shared with four specimens originating from the Meuse drainage basin (France), the Middle Rhine (Germany, 178 sequences) and one specimen from the Boyne River in Ireland. No haplotype was shared with specimens from the Seine. The second most represented haplotype in Corsica (H10) was shared with three specimens from the Upper Danube and the Middle Rhine (Germany). Concerning P. csikii, only one haplotype (H23) was detected in Corsica (seven sequences) and was shared with specimens from the Middle Rhine (Germany, 132 sequences) and the Po (Italy, two sequences).
COI haplotype networks obtained for the four Phoxinus species detected in Corsica, on the 745 sequences generated in this study and retrieved from GenBank (Table S1). Circle size is proportional to the observed haplotype frequencies and black points represent hypothetical haplotypes. Colors highlight drainage basins
Genetic diversity indices are given in Table S2. Comparison of genetic diversity parameters among the four species found in Corsica showed that P. septimaniae had the highest haplotype diversity, mean number of pairwise differences and nucleotide diversity while P. phoxinus had the lowest. The highest number of haplotypes was reported for P. csikii though only one was detected in Corsica. This species also showed the highest number of polymorphic sites. Conversely, the lowest number was reported for P. dragarum while this species had the highest number of haplotypes in Corsica and the lowest number of polymorphic sites.
Parasite diversity in Phoxinus minnows in Corsica
A total of six distinct parasites were recovered from Corsican Phoxinus minnows. Gyrodactylus spp. (Monogenea: Gyrodactylidae, recovered from all sampling localities) and a black spot disease-causing metacercariae, most likely Posthodiplostomum cuticola (Digenea: Diplostomidae, recovered from half the localities) were the two main parasites recovered from Corsica. Prevalence, mean abundance and mean intensity for both these two main parasites are reported for each locality and each host species (Tables 1, S3). P. cuticola metacercariae were recovered from all Phoxinus species except P. septimaniae and Gyrodactylus from all four species present in Corsica. Gyrodactylus species could not be identified at the species level due to the lack of matching sequences in GenBank, but our phylogenetic analysis showed the occurrence of four distinct clusters of Gyrodactylus sequences (Figs. 1, 3). The first cluster consists of Gyrodactylus recovered from a P. phoxinus from Tartagine river (Golo drainage) and from several P. dragarum from Fium’Orbo, Tavignano and Abatesco. Cluster 2 comprises a single sequence from a P. dragarum sampled in the Abatesco. The third cluster shows haplotypes being shared between a P. phoxinus from Vecchio river (Tavignano drainage basin) and from P. dragarum from the Asco (Golo drainage basin), Tavignano and Fium’Orbo rivers. The last cluster (Cluster 4) consists of two sequences originating from a P. dragarum from Corsigliese river (Tavignano drainage basin). Besides Gyrodactylus spp. and P. cuticola, several parasites could not be identified due to the low number of recovered individuals and development stages lacking diagnostic features. One Trematoda larvae was recorded from the body cavity of P. dragarum, Nematoda larvae were observed in the swimbladder of P. phoxinus, P. dragarum and P. septimaniae, two leeches (Hirudinea) were recovered from the skin of a P. phoxinus and a P. dragarum and a few freshwater mussel glochidia (Bivalvia: Unionidae) were recovered from the gills of three individuals P. dragarum, only in locality 9. The examination of brains did not show any D. phoxini metacercariae.
Phylogenetic tree inferred with MrBayes for the Gyrodactylus sampled in Corsica using ITS2 gene, with their corresponding host species. Numbers indicated in grey correspond to the four clusters. Minnows pictures come from Denys et al. (2020)
Influence of sampling localities on parasite communities
IndVal analysis did not show any preference of Gyrodactylus spp. nor black spot disease metacercariae towards a particular Phoxinus species and those results are thus not presented here. However, IndVal analysis showed that both black spot disease-causing metacercariae and Gyrodactylus spp. are characteristic of locality 3 (Tartagine river) as the IndVal as significantly higher for this locality (Table S4). Fidelity of Gyrodactylus spp. was very high whereas the specificity was intermediate as this taxon was recovered from all studied localities. Black spot disease metacercariae showed quite high specificity and intermediate fidelity. This corresponds to the locality showing the highest abundances for both parasites (Fig. 4).
Abundance of infection for a Gyrodactylus spp. and b Black spot disease (metacercariae) in Phoxinus minnows for each locality. Localities: (1) Moltifao, (2) Gavignano, (3) Castifao, (4) Barchetta, (5) Taglio-Isolaccio, (6) Venaco, (7) Altiani, (8) Piedicorte-di-Gaggio, (9) Saint-Georges, (10) Pancheraccia, (11) Ghisonaccia, (12) Prunelli-di-Fium’Orbo. Drainage basin A: Golo, B: Fium’Alto, C: Tavignano, D: Fium’Orbo, E: Abatesco. Boxplots appearing flat are due to the low prevalence of parasite in the corresponding locality
Discussion
Phoxinus minnows in Corsica: underestimated diversity and diverse introduction routes
Only one Phoxinus species was reported from Corsica (Roché 2001) until 2020 when Denys et al. (2020) showed not only the presence of P. phoxinus in the Golo river, but also of P. dragarum in the Tavignano river. The present study reports for the first time the occurrence of two additional species in Corsica: P. septimaniae and P. csikii. The results of the present study should however be analyzed taking into account the limitation of the molecular marker used. Hybridization events were reported for this genus (Palandačić et al. 2017, 2020, 2022; Corral-Lou et al. 2019). Our study used only a mitochondrial marker (COI) which does not allow the detection of eventual hybrids. However, as numerous Corsican localities present admixed populations, the presence of hybrids is likely. So, using a nuclear marker could be of interest in the future.
All four species of Phoxinus species introductions in Corsica are likely to result from their use as live bait by anglers. This mechanism has already been shown to be the cause of Phoxinus minnows introductions in other regions in Europe e.g. Norway and Portugal (Museth et al. 2007; Garcia-Raventós et al. 2020). This hypothesis is supported by the isolation of Corsica from the mainland by the Mediterranean Sea and the fact that Phoxinus minnows are not part of the native Corsican fish fauna (Changeux 1998; Roché 2001; Keith et al. 2020), indicating a human-mediated introduction and the common use of Phoxinus minnows as live bait for recreational fishing e.g. Salmo trutta angling (Banha et al. 2016). Two patterns of introduction are supported by the COI haplotype networks (Fig. 5): (1) In both northernmost Corsican drainage basins (Golo and Fium’Alto), P. phoxinus was the majority of sampled individuals, and P. csikii was only found in the Golo drainage basin, with haplotypes originating from the Middle Rhine, pointing toward an introduction through individuals from a continental Europe wholesaler and used as baits. This pattern resembles the case of P. csikii and P. phoxinus in Germany and the Netherlands (Palandačić et al. 2022). In France, minnows sold in all angling shops come from a same wholesaler (Amorvif; http://www.armorvif.com/) located in Brittany. Costedoat et al. (2014) characterized 50 specimens from this wholesaler and they found that 86% belong to the Meuse lineage of P. phoxinus, 6% were identified as P. csikii, 6% as Phoxinus fayollarum Denys, Dettai, Persat, Daszkiewicz, Hautecoeur and Keith, 2020 and 2% as P. septimaniae. The proportions of each species in both northernmost drainage basins (92% P. phoxinus and 8% P. csikii) add support to the hypothesis of a wholesaler-mediated introduction as the main species is the same in these localities and the wholesaler’s stock. The absence of two minor species P. septimaniae and P. fayollarum and presence of P. csikii would result from a random sampling that most likely occurred during the importation process. (2) In the southernmost drainages basins (Tavignano, Fium’Orbo and Abatesco), the occurrence of haplotypes from Southern France (Garonne, Mediterranean and Rhone) for P. septimaniae and P. dragarum could be indicative of angler-mediated introductions, as has been shown in the case of P. dragarum and Phoxinus bigerri Kottelat 2007 in the Iberian Peninsula (Corral-Lou et al. 2019). In this region, it is known that anglers travel widely and can use and introduce invasive species (Banha et al. 2016), and it is likely that anglers from continental France bring their live baits with them to be used in Corsican freshwaters.
Six species are recognized from continental France: P. phoxinus, P. csikii, P. septimaniae, P. dragarum, P. fayollarum and P. bigerri (Geiger et al. 2014; Corse et al. 2017; Palandačić et al. 2017; Schönhuth et al. 2018; Denys et al. 2020). To our knowledge, Phoxinus species reported from Italy consist of Phoxinus lumaireul (Schinz, 1840), P. septimaniae and P. csikii (Palandačić et al. 2017; De Santis et al. 2021) and those from Spain are P. bigerri, P. septimaniae and P. dragarum (Geiger et al. 2014; Corral-Lou et al. 2019; Keith et al. 2020). According to the extent of current knowledge concerning the distribution of this genus’ species, France is the only country where all species introduced to Corsica were reported. The known distribution of Phoxinus species in the countries closest to Corsica is thus an additional argument strengthening the hypothesis of multiple introductions from continental France. The inclusion of more individuals in haplotypes analysis would be of interest to better resolve haplotype networks as those generated in this study showed several hypothetical haplotypes and thus uncertain relationships.
Additionally, the study of these fishes’ parasites allows the hypothesis of secondary dispersion routes (Fig. 5). The observation of two shared Gyrodactylus clusters (clusters 1 and 3) between P. phoxinus from Golo drainage and P. dragarum from Tavignano and Fium’Orbo (plus Abatesco for one of them) drainages basins, combined with the absence of observation of P. dragarum in the Golo drainage basin, allows the hypothesis of a recreational anglers-mediated secondary dispersion of fish and their parasites from the Golo drainage to the Tavignano, Fium’Orbo and Abatesco drainages basins. As P. phoxinus was not observed in the Fium’Orbo and Abatesco rivers, a probable suite of events would be: a first transfer of P. phoxinus from the Golo drainage basin to the Tavignano drainage basin, and a second transfer of P. dragarum from the Tavignano drainage basin to the Abatesco and Fium’Orbo. The use of parasites as potential markers for introduction routes and historical distribution of hosts has already been discussed e.g. Kapentagyrus (Monogenea: Dactylogyridae) were used to clarify the origin of the Clupeidae Limnothrissa miodon (Boulenger, 1906) in Lake Kariba, Zimbabwe (Kmentová et al. 2019), the lack of Gyrodactylus on the round goby Neogobius melanostomus (Pallas, 1814) in Belgium suggested an introduction via ballast water (Huyse et al. 2015), host specific Dactylogyrus (Monogenea: Dactylogyridae) were used to evidence historical contact between North American and European Leuciscidae as well as contemporary contacts between these fish in North America (Šimková et al. 2022) and the host-specific Nippotaenia perccotti (Akhmerov, 1941) (Cestoda: Nippotaeniidae) proved useful to analyze the introduction vectors and dispersion pathways of its host, the Chinese sleeper Perccottus glenii Dybowski, 1877 (Reshetnikov et al. 2011). Again with regard to P. glenii, the Monogenea Gyrodactylus perccotti Ergens & Yukhimenko, 1973 has been used to suggest distinct introduction events in the Vistula and Danube drainages basins and a migration from the Vistula to the middle Dnieper River (Ondračková et al. 2012; Kvach et al. 2016). Here we show the usefulness of Gyrodactylus species as tags to elucidate regional-scale inter-basin transfer of their hosts in the case of Phoxinus minnows introduction in a small Mediterranean island.
Low parasite diversity of introduced Phoxinus minnows
In Eurasia, wild parasite communities of Phoxinus spp. show species richness ranging from four helminths reported from Frongoch lake in the UK (Bibby 1972) to 14 helminths species in the river Pechora, Russia (Dorovskikh and Stepanov 2008, 2009) and 14 parasite species including helminths and Copepoda in the rivers Chulman and Ungra, Russia (Boutorina and Reznik 2015). With five helminth species, the parasite richness of Phoxinus minnows in Corsica was thus among the lowest reported for this genus in Eurasia, along with two reports from the UK and one from a mountain water system in southern Norway (Ashworth and Bannerman 1927; Bibby 1972; Kristoffersen and Teigland 1997). A likely explanation for the low diversity reported from the UK is its insularity whereas the Phoxinus examined in southern Norway were qualified as recently spread. Corsica combines both these characteristics, being a small Mediterranean island where Phoxinus minnows are part of the non-native fauna, most likely introduced circa 2000 (Roché 2001). Several taxa known to occur in Phoxinus in mainland southern Europe were not recorded from Corsica, such as the Acanthocephala, the Argulidae (Ichthyostraca), the Allocreadiidae (Digenea) and the Cestoda (both Proteocephalidae and Diphyllobothridae) (Cruz et al. 2022). The few parasitological analyses of Phoxinus minnows conducted in mainland France showed radically distinct parasite communities, with the presence of Dactylogyrus spp., D. phoxini and Diplozoon spp., taxa not recorded in Corsica (Joyeux and Baer 1953; Euzet and Lambert 1971; Lambert 1977; Le Brun et al. 1988). However, it should be noted that these records originate from localities pertaining to the distribution ranges of P. fayollarum, a species not observed in Corsica, and of P. septimaniae, which was one of the least abundant Phoxinus in our samples and may have been introduced in small numbers Data concerning the parasite fauna of Phoxinus species found in Corsica are currently lacking.
The absence of record for D. phoxini in Corsica can appear surprising as this species was reported from Phoxinus minnows from Russia in the east to Spain in the west (Dorovskikh et al. 2008; Cruz et al. 2022). However, there are two possible explanations for this: (1) D. phoxini “missed the boat” and never reached Corsica i.e., the Phoxinus introduced to Corsica were devoid of this parasite, or (2) D. phoxini “drowned on arrival”, being unable to complete its life cycle.
As a side observation, there may still be much to unravel concerning Gyrodactylus diversity in Phoxinus. The four distinct clusters detected in the freshwaters of Corsica and highlighted by the use of ITS2 gene potentially correspond to four different species and two of them were shared between two species of hosts (P. phoxinus and P. dragarum) while the other two have only be found in P. dragarum. Due to technical difficulties, molecular and phylogenetic analysis could not be performed for Gyrodactylus specimens sampled on all Phoxinus species present in Corsica despite their occurrence on the four species recovered from the island. Numerous species of Gyrodactylus are known to occur on Phoxinus minnows throughout Eurasia (Harris et al. 2004; Bakke et al. 2007; Lumme et al. 2017), with some species recorded from a restricted number of sampling localities e.g., Gyrodactylus vimbi Shulman, 1954 (Finland) (Blazek et al. 2008), Gyrodactylus prostae Ergens, 1963, Gyrodactylus llewellyni Ergens & Dulmaa 1967 and Gyrodactylus minimus Malmberg, 1957 (Mongolia) (Ergens and Dulmaa 1967), and Gyrodactylus konovalovi Ergens 1976 (Russia) (Boutorina and Reznik 2015); and other species known from a wider geographical range e.g. Gyrodactylus macronychus Malmberg, 1957 (Czech Republic, Finland, Mongolia, Norway, Russia, the UK and Spain) (Ergens and Dulmaa 1967; Ergens 1976; Matějusová et al. 2000; Ziętara and Lumme 2003; Dorovskikh and Stepanov 2008; Grano-Maldonado et al. 2011; Pettersen et al. 2016; Cruz et al. 2022) or Gyrodactylus pannonicus Molnár, 1968 (Czech Republic, Finland, Russia, Slovakia and the UK) (Matějusová et al. 2000; Ziȩtara and Lumme 2002; Dorovskikh and Stepanov 2008; Blazek et al. 2008; Grano-Maldonado et al. 2011; Lumme et al. 2017). As Gyrodactylus is an extremely diversified genus, comprising both generalists and highly specialist species, with an estimated proportion of 30% infecting a single host (Bakke et al. 1992, 2007; Harris et al. 2004), it is likely that the diversity of introduced Gyrodactylus in Corsica is still underestimated. As an additional argument, this genus is known for its cryptic diversity resulting from the lack of morphological characters that would enable unambiguous species identification (Hansen et al. 2007; Razo-Mendivil et al. 2016; Ondračková et al. 2020; Rahmouni et al. 2023b, a). Studies of fish Monogenea regularly allow detection and description of new species of Gyrodactylus (e.g. Vanhove et al. 2011, 2014; Přikrylová et al. 2012a, b; Ziętara et al. 2012; Lumme et al. 2017; Shigoley et al. 2023; Zhang et al. 2023). More effort focused on the molecular identification of Gyrodactylus and their Phoxinus minnows hosts across Eurasia would likely reveal a considerable diversity which remains unknown for now. An example of this potential is the description of three new Gyrodactylus species from minnows sampled in the Baltic, White Sea and Black Sea basins, and in Mongolia (Lumme et al. 2017).
May introduced minnows transmit parasites to native species?
Black spot disease is caused by encysted metacercariae of Digenea, to which the fish host reacts by forming a fibrous capsule (Wittrock et al. 1991). Fish melanocytes are then attracted by these processes and melanin is deposited around the parasite, creating black spots visible to the naked eye (Tobler and Schlupp 2008). Though black spot disease-causing metacercariae observed in the present study could not be identified at the species level, there is a restricted number of Digenea known to cause such symptoms. Several species cause black spot disease in marine fish: Ichthyophaga sp. (Fecampiida: Piscinquilinidae) (Justine et al. 2009), Scaphanocephalus expansus (Creplin, 1842) and Scaphanocephalus sp. (Plagiorchiida: Heterophyidae) (Kohl et al. 2019; Dennis et al. 2019; Elmer et al. 2019; Cohen-Sánchez et al. 2023), and Cryptocotyle concava (Creplin, 1825) and Cryptocotyle lingua (Creplin, 1825) and Cryptocotyle jejuna (Nicoll, 1907) (Plagiorchiida: Opisthorchiidae) (Khan 2006; Aalvik et al. 2015; Duflot et al. 2021, 2023; Kornyychuk et al. 2022). Considering their environmental preferences, it is highly unlikely that the metacercariae found on Phoxinus minnows in Corsican freshwaters are any of these species. The genus Crassiphiala and Uvulifer (Diplostomida: Diplostomidae) are known to occur in American freshwaters (Berra and Au 1978; Quist et al. 2007; Tobler and Schlupp 2008; Wisenden et al. 2012; Achatz et al. 2019; López-Hernández et al. 2023) and Uvulifer sp. was detected in Northern Africa (Charo-Karisa et al. 2021). In Eurasia, black spot disease in fish has been attributed to Apophallus muehlingi (Jägerskiöld, 1899) and Apophallus donicus (Skrjabin and Lindtrop, 1919) (Plagiorchiida: Opisthorchiidae) (Sándor et al. 2017; Tyutin et al. 2023) but, to the extent of our knowledge, Posthodiplostomum cuticola (von Nordmann, 1832) (Diplostomida: Diplostomidae) is the most commonly reported agent of this condition in Europe (Shukhgalter and Chukalova 2002; Ondracková et al. 2004b, a, c; Zrnčić et al. 2009; Kirankaya and Ekmekçi 2011; Maja et al. 2012; Innal et al. 2020; Cech et al. 2021). As P. cuticola is also the only black spot disease-causing Digenea to have been reported from Phoxinus minnows including in continental France (Nicoll 1924; Kennedy 1974; Prouff 2017; Cruz et al. 2022), assuming that it is the species present in Corsica is a reasonable hypothesis. Unfortunately, parasitological data concerning Corsican fish anterior to this introduction of Phoxinus minnows on the island are not available, and it is thus not possible to know whether black spot disease was co-introduced with these fish. Black spot disease-causing Digenea, including P. cuticola, typically have an complex life cycle involving a piscivorous bird such as Ardeidae (Dönges 1964). Corsica being a Mediterranean island situated on bird migration routes (Bruderer and Liechti 1999; Jourdain et al. 2007; Maggini et al. 2020), the parasite could have been transported by migrating birds and have found competent fish hosts among the numerous species introduced in the island waterways, including Phoxinus minnows. While black spot disease does not seem to cause mortality in fish hosts, symptoms can include body deformation, muscle fiber necrosis and dysfunction of kidney and liver, which can be particularly harmful to fry (Williams 1994; Marković and Krsmanović 2008; Innal et al. 2020). This pathogenicity could be a point of concern as a preliminary study conducted in 2021 allowed detection of the presence in locality 3 (Tartagine river) of black spot disease-causing metacercariae on brown trout Salmo trutta Linnaeus, 1758, a species considered of patrimonial interest in Corsica. This disease had not been detected in S. trutta in Corsica before the present study despite extensive surveys of this species’ parasitofauna (Quilichini et al. 2007; Quilchini et al. 2010), and, to the extent of our knowledge, P. cuticola was only reported once from S. trutta, in Poland (Rolbiecki et al. 2009).
Conclusion
Although the extensive range of ecological consequences of Phoxinus minnows introduction in Corsica is not yet fully understood, their presence in sustainable populations is of concern as they could compete with native fish. Phoxinus minnows are reported to impact native fish e.g. reduced recruitment and growth rates in S. trutta in Scandinavia, possibly resulting from competition for trophic resources as there is a dietary overlap between Phoxinus minnows and young S. trutta (Museth et al. 2007, 2010). It has been suggested that the harsh Mediterranean climatic conditions could limit the establishment of introduced species having evolved under different environmental conditions (Filipe et al. 2010), but Phoxinus minnows seem to have broad-enough ecological tolerances to successfully colonize this type of habitat.
The introduction of Phoxinus minnows has likely had parasitological impacts on native species, as shown by the case of black spot disease. While it is not possible to know whether these fish co-introduced the parasite responsible for this infection, its occurrence in Corsican freshwaters is most likely favorable to the disease’s persistence on the island, and thus its potential transmission to native species. Parasites, especially Monogenea with relatively restricted host spectrum, can be useful markers of small-scale secondary introduction patterns, and allow detection of inter-basin transfer of hosts at the regional scale.
In the context of the European Water Framework Directive, all member states of the European Union have to monitor their fish populations. It has been shown that Phoxinus species are reliably identifiable on the basis of the nuptial coloration pattern only during their spawning period (Denys et al. 2020). As the monitoring periods of fish populations are not always compatible with the Phoxinus spawning period, the addition of molecular identification (i.e. barcoding) in the context of the Water Framework Directive should be considered. A complementary approach could be the use of eDNA detection methods (Pont et al. 2021).
Another primordial aspect of Phoxinus minnows’ introductions is the importance of applying management plans able to prevent future introductions. A Europe-wide ban on live bait fishing could be one approach to prevent future fish introductions, especially in other Mediterranean islands that currently seem free of Phoxinus minnows, such as Sardinia and Sicily (Orrù et al. 2010; Marrone and Naselli-Flores 2015).
Data availability
The data supporting the findings of this study are available within the article and its supplementary material. Raw data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Aalvik I, Moland E, Olsen E, Stenseth N (2015) Spatial ecology of coastal Atlantic cod Gadus morhua associated with parasite load. J Fish Biol 87:449–464. https://doi.org/10.1111/jfb.12731
Achatz T, Pulis E, Fecchio A et al (2019) Phylogenetic relationships, expanded diversity and distribution of Crassiphiala spp. (Digenea, Diplostomidae), agents of black spot disease in fish. Parasitol Res 118:2781–2787. https://doi.org/10.1007/s00436-019-06439-y
Allen C, Briano JA, Varone L et al (2010) Exploitation of a high genomic mutation rate in Solenopsis invicta virus 1 to infer demographic information about its host, Solenopsis invicta. J Invertebr Pathol 105:105–111. https://doi.org/10.1016/j.jip.2010.05.018
Artaev ON, Turbanov IS, Bolotovskiy AA et al (2024) Taxonomic revision of Phoxinus minnows (Leuciscidae) from Caucasus, with description of a new narrow-ranged endemic species. Zoosyst Evol 100:291–308. https://doi.org/10.3897/zse.100.115696
Ashworth JH, Bannerman JCW (1927) On a Tetracotyle (T. phoxini) in the Brain of the Minnow. Trans R Soc Edinb 55:159–172. https://doi.org/10.1017/S008045680001629X
Bakke TA, Harris PD, Jansen PA, Hansen LP (1992) Host specificity and dispersal strategy in gyrodactylid monogeneans, with particular reference to Gyrodactylus salaris (Platyhelminthes, Monogenea). Dis Aquat Org 13:63–74. https://doi.org/10.3354/dao013063
Bakke TA, Cable J, Harris PD (2007) The biology of gyrodactylid monogeneans: The “Russian-Doll Killers.” In: Baker JR, Muller R, Rollinson D (eds) Advances in parasitology. Academic Press, pp 161–460
Bandelt HJ, Forster P, Röhl A (1999) Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol 16:37–48. https://doi.org/10.1093/oxfordjournals.molbev.a026036
Banha F, Diniz AM, Anastácio P (2016) The role of angler’s perceptions and habits in biological invasions: perspectives from Iberian Peninsula. Aquat Conser Mar Freshw Ecosyst 27:51–64. https://doi.org/10.1002/aqc.2677
Bayçelebi E, Aksu İ, Turan D (2024) Description of a new species of Phoxinus from the Ergene River (Aegean Sea Basin) in Türkiye (Actinopterygii, Leuciscidae). Zoosystematics Evol 100:101–110. https://doi.org/10.3897/zse.100.113467
Behrens-Chapuis S, Herder F, Esmaeili HR et al (2015) Adding nuclear rhodopsin data where mitochondrial COI indicates discrepancies—Can this marker help to explain conflicts in cyprinids? DNA Barcodes 3:187–199. https://doi.org/10.1515/dna-2015-0020
Behrens-Chapuis S, Herder F, Geiger MF (2021) Adding DNA barcoding to stream monitoring protocols—What’s the additional value and congruence between morphological and molecular identification approaches? PLoS ONE 16:e0244598. https://doi.org/10.1371/journal.pone.0244598
Benovics M, Vukić J, Šanda R et al (2020) Disentangling the evolutionary history of peri-Mediterranean cyprinids using host-specific gill monogeneans. Int J Parasitol 50:969–984. https://doi.org/10.1016/j.ijpara.2020.05.007
Berg LS (1949) Ryby presnykh vod SSSR i sopredelʹnykh stran [Freshwater fishes of the U.S.S.R. and adjacent countries]. In: 4th ed. Israel Program for Scientific Translations, Jerusalem
Berra T, Au R (1978) Incidence of black spot disease in fishes in Cedar Fork Creek, Ohio. Ohio J Sci 78:318–322
Bianco PG (2014) An update on the status of native and exotic freshwater fishes of Italy. J Appl Ichthyol 30:62–77. https://doi.org/10.1111/jai.12291
Bianco PG, De Bonis S (2015) A taxonomic study on the genus Phoxinus (Actinopterygi, Cyprinidae) from Italy and western Balkans with description of four new species: P. ketmaieri, P. karsticus, P. apollonicus and P. likai. Res Wildl Cons 4:1–17
Bibby MC (1972) Population biology of the helminth parasites of Phoxinus phoxinus (L.), the minnow, in a Cardiganshire lake. J Fish Biol 4:289–300. https://doi.org/10.1111/j.1095-8649.1972.tb05677.x
Bickford D, Lohman DJ, Sodhi NS et al (2007) Cryptic species as a window on diversity and conservation. Trends Ecol Evol 22:148–155. https://doi.org/10.1016/j.tree.2006.11.004
Biek R, Drummond AJ, Poss M (2006) a virus reveals population structure and recent demographic history of its carnivore host. Science 311:538–541. https://doi.org/10.1126/science.1121360
Blazek RD, Bagge A, Valtonen ET (2008) Monogenean assemblages and the apparent transmission capability of monogeneans between related fish species: an experimental study. Parasitol Res 102:1359–1366. https://doi.org/10.1007/s00436-008-0918-3
Bogutskaya NG, Jelić D, Vucić M et al (2020) Description of a new species of Phoxinus from the upper Krka River (Adriatic Basin) in Croatia (Actinopterygii: Leuciscidae), first discovered as a molecular clade. J Fish Biol 96:378–393. https://doi.org/10.1111/jfb.14210
Bogutskaya NG, Diripasko OA, Palandačić A (2023) Novel data support validity of Phoxinus chrysoprasius (Pallas, 1814) (Actinopterygii, Leuciscidae). Eur J Taxon 861:1–20. https://doi.org/10.5852/ejt.2023.861.2061
Boutorina TE, Reznik IV (2015) Biological characteristic of Phoxinus phoxinus L. in Chulman and Ungra rivers (Southern Yakutia). Contemp Probl Ecol 8:56–64. https://doi.org/10.1134/S1995425515010011
Bruderer B, Liechti F (1999) Bird migration across the Mediterranean. In: Proceedings of international ornithological congress Durban. Johannesburg: BirdLife South Africa. pp 1983–1999
Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83:575–583. https://doi.org/10.2307/3284227
Cambray JA (2003) Impact on indigenous species biodiversity caused by the globalisation of alien recreational freshwater fisheries. Hydrobiologia 500:217–230. https://doi.org/10.1023/A:1024648719995
Cech G, Sándor D, Molnár K et al (2021) Digenean trematodes in Hungarian freshwater aquacultures. Food Waterborne Parasitol 22:e00101. https://doi.org/10.1016/j.fawpar.2020.e00101
Changeux T (1998) Insular characteristics of freshwater fish communities in the island of Corsica, comparison with French continental coastal rivers. Ital J Zool 65:305–311. https://doi.org/10.1080/11250009809386838
Charleston MA, Perkins SL (2006) Traversing the tangle: algorithms and applications for cophylogenetic studies. J Biomed Inform 39:62–71. https://doi.org/10.1016/j.jbi.2005.08.006
Charo-Karisa H, Ali S, Marijani E et al (2021) Genetic parameters for black spot disease (diplopstomiasis) caused by Uvulifer sp infection in Nile tilapia (Oreochromis niloticus L.). Aquaculture 532:736039. https://doi.org/10.1016/j.aquaculture.2020.736039
Chen X (1988) A new species of Phoxinus from China (Pisces, Cypriniformes). Sinozoologia 6:35–38
Clavero M, Garcia-Berthou E (2005) Invasive species are a leading cause of animal extinctions. Trends Ecol Evol 20:110–110. https://doi.org/10.1016/j.tree.2005.01.003
Clayton DH, Johnson KP (2003) Linking coevolutionary history to ecological process: doves and lice. Evolution 57:2335–2341. https://doi.org/10.1111/j.0014-3820.2003.tb00245.x
Cohen-Sánchez A, María Valencia J, Box A et al (2023) Black spot disease related to a trematode ectoparasite causes oxidative stress in Xyrichtys novacula. J Exp Mar Biol Ecol 560:151854. https://doi.org/10.1016/j.jembe.2022.151854
Corral-Lou A, Perea S, Aparicio E, Doadrio I (2019) Phylogeography and species delineation of the genus Phoxinus Rafinesque, 1820 (Actinopterygii: Leuciscidae) in the Iberian Peninsula. J Zoolog Syst Evol Res 57:926–941. https://doi.org/10.1111/jzs.12320
Corse E, Meglecz E, Archambaud G et al (2017) A from-benchtop-to-desktop workflow for validating HTS data and for taxonomic identification in diet metabarcoding studies. Mol Ecol Resour 17:e146–e159. https://doi.org/10.1111/1755-0998.12703
Costedoat C, Grenier R, Chappaz R, et al (2014) Révision de la taxonomie ichtyologique en métropole : validation moléculaire du guide de détermination et investigation des aires de répartition. ONEMA-IMBE
Criscione CD, Cooper B, Blouin MS (2006) Parasite genotypes identify source populations of migratory fish more accurately than fish genotypes. Ecology 87:823–828. https://doi.org/10.1890/0012-9658(2006)87[823:PGISPO]2.0.CO;2
Cruz A, Llinares C, Martín-Barrio I et al (2022) Comparing morphological, parasitological, and genetic traits of an invasive minnow between intermittent and perennial stream reaches. Freshw Biol 67:2035–2049. https://doi.org/10.1111/fwb.13994
De Santis V, Delmastro GB, Vanetti I et al (2021) Species composition of introduced and natural minnow populations of the Phoxinus cryptic complex in the westernmost part of the Po River Basin (north Italy). Biol Invasions 23:657–668. https://doi.org/10.1007/s10530-020-02406-2
Dennis M, Izquierdo A, Conan A et al (2019) Scaphanocephalus-associated dermatitis as the basis for black spot disease in Acanthuridae of St. Kitts. West Indies Dis Aquat Org 137:53–63. https://doi.org/10.3354/dao03419
Denys GPJ, Manne S (2019) First record of Phoxinus csikii Hankó 1922 (Actinopterygii, Cypriniformes) in France. Cybium 43:199–202. https://doi.org/10.26028/CYBIUM/2019-423-008
Denys GPJ, Dettai A, Persat H et al (2020) Revision of Phoxinus in France with the description of two new species (Teleostei, Leuciscidae). Cybium 44:205–237. https://doi.org/10.26028/CYBIUM/2020-443-003
Dönges J (1964) Der Lebenszyklus von Posthodiplostomum cuticola (v. Nordmann 1832) Dubois 1936 (Trematoda, Diplostomatidae). Z F Parasitenkunde 24:169–248. https://doi.org/10.1007/BF00259550
Donlan CJ, Wilcox C (2008) Diversity, invasive species and extinctions in insular ecosystems. J Appl Ecol 45:1114–1123. https://doi.org/10.1111/j.1365-2664.2008.01482.x
Dorovskikh GN, Stepanov VG (2008) Change in the structure of component parasite communities with host age. Rus J Ecol 39:215. https://doi.org/10.1134/S1067413608030119
Dorovskikh GN, Stepanov VG (2009) Structure of component parasite communities in the grayling, Thymallus thymallus L. (Salmoniformes, Thymallidae), and minnow, Phoxinus phoxinus L.(Cypriniformes, Cyprinidae), from the upper reaches of the Pechora River. Biol Bull 36:298–306. https://doi.org/10.1134/S1062359009030108
Dorovskikh GN, Stepanov VG, Golikova EA, Vostrikova AV (2008) Component parasite communities in the minnow Phoxinus phoxinus (L.) from ecologically safe and polluted reservoirs. Parazitologiya 42:280–291
Duflot M, Gay M, Midelet G et al (2021) Morphological and molecular identification of Cryptocotyle lingua metacercariae isolated from Atlantic cod (Gadus morhua) from Danish seas and whiting (Merlangius merlangus) from the English Channel. Parasitol Res 120:3417–3427. https://doi.org/10.1007/s00436-021-07278-6
Duflot M, Cresson P, Julien M et al (2023) Black spot diseases in seven commercial fish species from the english channel and the North Sea: infestation levels, identification and population genetics of Cryptocotyle spp. Parasite 30:28. https://doi.org/10.1051/parasite/2023028
Dufrêne M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67:345–366. https://doi.org/10.1890/0012-9615(1997)067[0345:SAAIST]2.0.CO;2
Dyldin YuV, Orlov AM, Hanel L et al (2023) Ichthyofauna of the fresh and brackish waters of Russia and adjacent areas: annotated list with taxonomic comments. 2. Order cypriniformes, suborders catostomoidei. Cobitoidei and Cyprinoidei J Ichthyol 63:636–686. https://doi.org/10.1134/S0032945223040045
Ellender B, Weyl O (2014) A review of current knowledge, risk and ecological impacts associated with non-native freshwater fish introductions in South Africa. Aquat Invasions 9:117–132. https://doi.org/10.3391/ai.2014.9.2.01
Elmer F, Kohl Z, Johnson P, Peachey R (2019) Black spot syndrome in reef fishes: using archival imagery and field surveys to characterize spatial and temporal distribution in the Caribbean. Coral Reefs 38:1303–1315. https://doi.org/10.1007/s00338-019-01843-3
Ergens R (1976) Variability of hard parts of opisthaptor of two species of Gyrodactylus nordmann, 1832 (Monogenoidea) from Phoxinus Phoxinus (L.). Folia Parasitol (praha) 23:111–126
Ergens R, Dulmaa A (1967) Monogenoidea from the genus Phoxinus (Cyprinidae) from Mongolia. Folia Parasitol 14:321–333
Euzet L, Lambert A (1971) Compléments à l’étude de la larve de Diplozoon paradoxum Von Nordmann, 1832 (Monogenea). Ann Parasitol Hum Comp 46:675–684. https://doi.org/10.1051/parasite/1971466675
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791. https://doi.org/10.1111/j.1558-5646.1985.tb00420.x
Filipe AF, Filomena Magalhães M, Collares-Pereira MJ (2010) Native and introduced fish species richness in Mediterranean streams: the role of multiple landscape influences. Diversity Distrib 16:773–785. https://doi.org/10.1111/j.1472-4642.2010.00678.x
Gagne RB, Crooks KR, Craft ME et al (2022) Parasites as conservation tools. Conserv Biol 36:e13719. https://doi.org/10.1111/cobi.13719
Garcia-Raventós A, Martins FMS, Teixeira A et al (2020) Origin and history of Phoxinus (Cyprinidae) introductions in the Douro Basin (Iberian Peninsula): an update inferred from genetic data. Biol Invasions 22:2409–2419. https://doi.org/10.1007/s10530-020-02279-5
Geiger MF, Herder F, Monaghan MT et al (2014) Spatial heterogeneity in the Mediterranean biodiversity hotspot affects barcoding accuracy of its freshwater fishes. Mol Ecol Res 14:1210–1221. https://doi.org/10.1111/1755-0998.12257
Goedknegt MA, Feis ME, Wegner KM et al (2016) Parasites and marine invasions: ecological and evolutionary perspectives. J Sea Res 113:11–27. https://doi.org/10.1016/j.seares.2015.12.003
Grano-Maldonado MI, Bron JE, Longshaw M, Shinn AP (2011) The accidental transfer of Gyrodactylus (Monogenea) during short duration fish transportation. Fish Pathol 46:71–79. https://doi.org/10.3147/jsfp.46.71
Hansen H, Bakke TA, Bachmann L (2007) DNA taxonomy and barcoding of monogenean parasites: lessons from Gyrodactylus. Trends Parasitol 23:363–367. https://doi.org/10.1016/j.pt.2007.06.007
Harris PD, Shinn AP, Cable J, Bakke TA (2004) Nominal species of the genus Gyrodactylus von Nordmann 1832 (Monogenea: Gyrodactylidae), with a list of principal host species. Syst Parasitol 59:1–27. https://doi.org/10.1023/B:SYPA.0000038447.52015.e4
Hebert PDN, Ratnasingham S, de Waard JR (2003) Barcoding animal life: cytochrome c oxidase subunit 1 divergences among closely related species. Proc R Soc b: Biol Sci 270:S96–S99. https://doi.org/10.1098/rsbl.2003.0025
Huyse T, Vanhove MPM, Mombaerts M et al (2015) Parasite introduction with an invasive goby in Belgium double trouble. Parasitol Res 114:2789–2793. https://doi.org/10.1007/s00436-015-4544-6
Innal D, Özdemir F, Stavrescu-Bedivan M et al (2020) Occurrence of black spot disease induced by Posthodiplostomum cuticola (Nordmann, 1832) (Digenea: Diplostomatidae) in endemic and native fish of Turkey: seven new host records. J Hellenic Vet Med Soc 71:2121–2126
Jourdain E, Gauthier-Clerc M, Bicout D, Sabatier P (2007) Bird migration routes and risk for pathogen dispersion into Western Mediterranean Wetlands. Emerg Infect Dis 13:365. https://doi.org/10.3201/eid1303.060301
Joyeux C, Baer J-G (1953) Sur quelques Helminthes de la région de Gannat (Allier). Publ Soc Linn Lyon 22:25–32. https://doi.org/10.3406/linly.1953.7547
Justine J, Leblanc P, Keller F, Lester R (2009) Turbellarian black spot disease in bluespine unicornfish Naso unicornis in New Caledonia, caused by the parasitic turbellarian Piscinquilinus sp. Dis Aquat Org 85:245–249. https://doi.org/10.3354/dao02082
Katoh K, Misawa K, Kuma K, Miyata T (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast fourier transform. Nucleic Acids Res 30:3059–3066. https://doi.org/10.1093/nar/gkf436
Keith P, Poulet N, Denys G, et al (2020) Les Poissons d’eau douce de France - 2ème édition. Biotope Editions, Mèze ; Muséum national d’Histoire Naturelle, Paris
Kennedy CR (1974) A checklist of British and Irish freshwater fish parasites with notes on their distribution. J Fish Biol 6:613–644. https://doi.org/10.1111/j.1095-8649.1974.tb05104.x
Khan R (2006) Assessment of stress-related bioindicators in winter flounder (Pleuronectes americanus) exposed to discharges from a pulp and paper mill in Newfoundland: a 5-year field study. Arch Environ Contam Toxicol 51:103–110. https://doi.org/10.1007/s00244-005-0166-9
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120. https://doi.org/10.1007/BF01731581
Kirankaya S, Ekmekçi F (2011) Frequency of black spot disease in Cobitis cf. turcica from Pinarbasi Springs (Haymana, Turkey). Folia Zool 60:350–354
Kmentová N, Van Steenberge M, Thys van den Audenaerde DFE et al (2019) Co-introduction success of monogeneans infecting the fisheries target Limnothrissa miodon differs between two non-native areas: the potential of parasites as a tag for introduction pathway. Biol Invasions 21:757–773. https://doi.org/10.1007/s10530-018-1856-3
Knebelsberger T, Dunz AR, Neumann D, Geiger MF (2015) Molecular diversity of Germany’s freshwater fishes and lampreys assessed by DNA barcoding. Mol Ecol Resour 15:562–572. https://doi.org/10.1111/1755-0998.12322
Kohl Z, Calhoun D, Elmer F et al (2019) Black-spot syndrome in Caribbean fishes linked to trematode parasite infection (Scaphanocephalus expansus). Coral Reefs 38:917–930. https://doi.org/10.1007/s00338-019-01819-3
Kornyychuk Y, Polyakova T, Pronkina N (2022) New data on pipefishes’ and seahorse’s endohelminths off Crimean coasts of the Black Sea. Helminthologia 59:74–82. https://doi.org/10.2478/helm-2022-0006
Kottelat M (2007) Three new species of Phoxinus from Greece and southern France (Teleostei: Cyprinidae). Ichthyol Explor Freshwaters 18:145
Kottelat M (2006) Fishes of Mongolia. A check-list of the fishes known to occur in Mongolia with comments on systematics and nomenclature. The World Bank, Washington
Kristoffersen B, Teigland L (1997) Parasites of minnow (Phoxinus phoxinus) from a mountain water system of southern Norway. XVIII Symposium of the Scandinavian Society for Parasitology, Bornholm, pp 78–79
Kumar S, Stecher G, Li M et al (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. https://doi.org/10.1093/molbev/msy096
Kvach Y, Kutsokon Y, Stepien CA, Markovych M (2016) Role of the invasive Chinese sleeper Perccottus glenii (Actinopterygii: Odontobutidae) in the distribution of fish parasites in Europe: new data and a review. Biologia 71:941–951. https://doi.org/10.1515/biolog-2016-0112
Lambert A (1977) Les Monogènes Monopisthocotylea parasites des Poissons d’eau douce de la France méditerranéenne. Bull Mus Natl Hist Nat Ser 3(429):177–214
Lambert A (1997) Introduction de poissons dans les milieux aquatiques continentaux : «Quid de leurs parasites?». Bull Fr Pêche Piscic. https://doi.org/10.1051/kmae:1997032
Lanfear R, Calcott B, Ho SYW, Guindon S (2012) PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol Biol Evol 29:1695–1701. https://doi.org/10.1093/molbev/mss020
Le Brun N, Renaud F, Lambert A (1988) The genus Diplozoon (Monogenea, Polyopisthocotylea) in southern France: speculation and specificity. Int J Parasitol 18:395–400. https://doi.org/10.1016/0020-7519(88)90150-6
López-Hernández D, Leibowitz M, Pinto H, Leal C (2023) First report of Crassiphiala sp. (Trematoda: Diplostomidae) as an etiological agent of black spot disease in commercial ornamental fish from Brazil. Parasitol Res 122:1037–1042. https://doi.org/10.1007/s00436-023-07794-7
Lumme J, Mäkinen H, Ermolenko AV et al (2016) Displaced phylogeographic signals from Gyrodactylus arcuatus, a parasite of the three-spined stickleback Gasterosteus aculeatus, suggest freshwater glacial refugia in Europe. Int J Parasitol 46:545–554. https://doi.org/10.1016/j.ijpara.2016.03.008
Lumme J, Ziętara MS, Lebedeva D (2017) Ancient and modern genome shuffling: reticulate mito-nuclear phylogeny of four related allopatric species of Gyrodactylus von Nordmann, 1832 (Monogenea: Gyrodactylidae), ectoparasites on the Eurasian minnow Phoxinus phoxinus (L.)(Cyprinidae). Systematic parasitology 94:183–200. https://doi.org/10.1007/s11230-016-9696-y
Maggini I, Cardinale M, Sundberg JH et al (2020) Recent phenological shifts of migratory birds at a Mediterranean spring stopover site: species wintering in the Sahel advance passage more than tropical winterers. PLoS ONE 15:e0239489. https://doi.org/10.1371/journal.pone.0239489
Maja M, Cirkovic M, Nevenka A et al (2012) Posthodiplostomatosis in a fishpond in Serbia. Acta Vet (beogr) 62:101–109. https://doi.org/10.2298/AVB1201101M
Marković G, Krsmanović M (2008) The influence of Posthodiplostomum cuticola (Digenea, Trematodes) metacercariae infestation on the growth rate of Leuciscus cephalus L. (Cyprinidae, Pisces). Acta Agric Serb 13:73–76
Marrone F, Naselli-Flores L (2015) A review on the animal xenodiversity in Sicilian inland waters (Italy). Adv Oceanogr Limnol 6:2–12. https://doi.org/10.4081/aiol.2015.5451
Matějusová I, Morand S, Gelnar M (2000) Nestedness in assemblages of gyrodactylids (Monogenea: Gyrodactylidea) parasitising two species of cyprinid – with reference to generalists and specialists. Int J Parasitol 30:1153–1158. https://doi.org/10.1016/S0020-7519(00)00097-7
Matejusová I, Gelnar M, McBeath AJ et al (2001) Molecular markers for gyrodactylids (Gyrodactylidae: Monogenea) from five fish families (Teleostei). Int J Parasit 31:738–745. https://doi.org/10.1016/S0020-7519(01)00176-X
Milardi M, Aschonitis V, Gavioli A et al (2018) Run to the hills: exotic fish invasions and water quality degradation drive native fish to higher altitudes. Sci Total Environ 624:1325–1335. https://doi.org/10.1016/j.scitotenv.2017.12.237
Miró A, Ventura M (2015) Evidence of exotic trout mediated minnow invasion in Pyrenean high mountain lakes. Biol Invasions 17:791–803. https://doi.org/10.1007/s10530-014-0769-z
Mitrofanov V, Dukravec G, Sudorova A, Soloninova L (1987) Ryby Kazakhstana [Fishes of Kazakhstan] Tom. 2. Alma-Ata: Nauka
Mouillot D, Culioli J-M, Chi TD (2002) Indicator species analysis as a test of non-random distribution of species in the context of marine protected areas. Environ Conserv 29:385–390. https://doi.org/10.1017/S0376892902000267
Müller G (1995) Prevalence and abundance of two trematode parasites, Diplostomum phoxini and Macrolecithus papilliger in European minnows (Phoxinus phoxinus) in an artificial Swiss Alpine lake. Aquatic Sci 57:119–126. https://doi.org/10.1007/BF00877380
Museth J, Hesthagen T, Sandlund OT et al (2007) The history of the minnow Phoxinus phoxinus (L.) in Norway: from harmless species to pest. J Fish Biol 71:184–195. https://doi.org/10.1111/j.1095-8649.2007.01673.x
Museth J, Borgstrøm R, Brittain JE (2010) Diet overlap between introduced European minnow (Phoxinus phoxinus) and young brown trout (Salmo trutta) in the lake, Øvre Heimdalsvatn: a result of abundant resources or forced niche overlap? In: Brittain JE, Borgstrøm R (eds) The subalpine lake ecosystem, Øvre Heimdalsvatn, and its catchment: local and global changes over the last 50 years. Springer, Netherlands, Dordrecht, pp 93–100
Nicoll W (1924) A reference list of the trematode parasites of British freshwater fishes. Parasitology 16:127–144. https://doi.org/10.1017/S0031182000019971
Nieberding CM, Olivieri I (2007) Parasites: proxies for host genealogy and ecology? Trends Ecol Evol 22:156–165. https://doi.org/10.1016/j.tree.2006.11.012
Nieberding C, Morand S, Libois R, Michaux JR (2004) A parasite reveals cryptic phylogeographic history of its host. Proc R Soc B Biol Sci 271:2559–2568. https://doi.org/10.1098/rspb.2004.2930
Nieberding C, Morand S, Libois R, Michaux JR (2006) Parasites and the island syndrome: the colonization of the western Mediterranean islands by Heligmosomoides polygyrus (Dujardin, 1845). J Biogeogr 33:1212–1222. https://doi.org/10.1111/j.1365-2699.2006.01503.x
Ondracková M, Bartosová S, Valová Z et al (2004a) Occurrence of black-spot disease caused by metacercariae of Posthodiplostomum cuticola among juvenile fishes in water bodies in the Morava River basin. Acta Parasitol 49:222–227
Ondracková M, Reichard M, Jurajda P, Gelnar M (2004b) Seasonal dynamics of Posthodiplostomum cuticola (Digenea, Diplostomatidae) metacercariae and parasite-enhanced growth of juvenile host fish. Parasitol Res 93:131–136. https://doi.org/10.1007/s00436-004-1123-7
Ondracková M, Simková A, Gelnar M, Jurajda P (2004c) Posthodiplostomum cuticola (Digenea: Diplostomatidae) in intermediate fish hosts: factors contributing to the parasite infection and prey selection by the definitive bird host. Parasitology 129:761–770. https://doi.org/10.1017/S0031182004006456
Ondračková M, Matejusová I, Grabowska J (2012) Introduction of Gyrodactylus perccotti (Monogenea) into Europe on its invasive fish host, Amur sleeper (Perccottus glenii, Dybowski 1877). Helminthologia 49:21–26. https://doi.org/10.2478/s11687-012-0004-3
Ondračková M, Seifertová M, Bryjová A et al (2020) Morphometric and genetic evidence for cryptic diversity in Gyrodactylus (Monogenea) infecting non-native European populations of Ameiurus nebulosus and A. melas. Parasitology 147:1700–1711. https://doi.org/10.1017/S0031182020001195
Orrù F, Deiana AM, Cau A (2010) Introduction and distribution of alien freshwater fishes on the island of Sardinia (Italy): an assessment on the basis of existing data sources. J Appl Ichthyol 26:46–52. https://doi.org/10.1111/j.1439-0426.2010.01501.x
Page RDM (2003) Tangled trees: phylogeny, cospeciation, and coevolution. University of Chicago Press, Chicago
Palandačić A, Naseka A, Ramler D, Ahnelt H (2017) Contrasting morphology with molecular data: an approach to revision of species complexes based on the example of European Phoxinus (Cyprinidae). BMC Evol Biol 17:184. https://doi.org/10.1186/s12862-017-1032-x
Palandačić A, Kruckenhauser L, Ahnelt H, Mikschi E (2020) European minnows through time: museum collections aid genetic assessment of species introductions in freshwater fishes (Cyprinidae: Phoxinus species complex). Heredity 124:410–422. https://doi.org/10.1038/s41437-019-0292-1
Palandačić A, Witman K, Spikmans F (2022) Molecular analysis reveals multiple native and alien Phoxinus species (Leusciscidae) in the Netherlands and Belgium. Biol Invasions 24:2273–2283. https://doi.org/10.1007/s10530-022-02784-9
Pariselle A, Boeger WA, Snoeks J et al (2011) The Monogenean parasite fauna of Cichlids: a potential tool for host biogeography. Int J Evol Biol 2011:e471480. https://doi.org/10.4061/2011/471480
Pettersen RA, Ostbye K, Holmen J et al (2016) Gyrodactylus spp. diversity in native and introduced minnow (Phoxinus phoxinus) populations: no support for “the enemy release” hypothesis. Parasites Vectors 9:51. https://doi.org/10.1186/s13071-016-1306-y
Pont D, Valentini A, Rocle M et al (2021) The future of fish-based ecological assessment of European rivers: from traditional EU water framework directive compliant methods to eDNA metabarcoding-based approaches. J Fish Biol 98:354–366. https://doi.org/10.1111/jfb.14176
Prenter J, MacNeil C, Dick JTA, Dunn AM (2004) Roles of parasites in animal invasions. Trends Ecol Evol 19:385–390. https://doi.org/10.1016/j.tree.2004.05.002
Přikrylová I, Blažek R, Vanhove MPM (2012a) An overview of the Gyrodactylus (Monogenea: Gyrodactylidae) species parasitizing African catfishes, and their morphological and molecular diversity. Parasitol Res 110:1185–1200. https://doi.org/10.1007/s00436-011-2612-0
Přikrylová I, Blažek R, Gelnar M (2012b) Gyrodactylus malalai sp. nov. (Monogenea, Gyrodactylidae) from Nile tilapia, Oreochromis niloticus (L.) and Redbelly tilapia, Tilapia zillii (Gervais) (Teleostei, Cichlidae) in the Lake Turkana. Kenya Acta Parasitol 57:122–130. https://doi.org/10.2478/s11686-012-0017-6
Prouff B (2017) Application des codes pathologie lors des inventaires piscicoles réalisés dans les cours d’eau du département du Tarn. Fédération du Tarn pour la Pêche et la protection du milieu aquatique, Castres
Quilchini Y, Foata J, Mouillot D et al (2010) The influence of altitude, hydrographic network and season on brown trout parasites in Corsica using indicator species analysis. J Helminthol 84:13–19. https://doi.org/10.1017/S0022149X09990101
Quilichini Y, Foata J, Orsini A et al (2007) Parasitofauna study of the brown trout, Salmo trutta (Pisces, Teleostei) from Corsica (Mediterranean island) rivers. Parasite 14:257–260. https://doi.org/10.1051/parasite/2007143257
Quist M, Bower M, Hubert W (2007) Infection by a black spot-causing species of Uvulifer and associated opercular alterations in fishes from a high-desert stream in Wyoming. Dis Aquat Org 78:129–136. https://doi.org/10.3354/dao01875
Rahmouni C, Seifertová M, Benovics M, Šimková A (2023) Diversity and Phylogeny of Gyrodactylus spp. (Monogenea: Gyrodactylidae) across the Strait of Gibraltar: parasite speciation and historical biogeography of West Mediterranean Cyprinid hosts. Diversity 15:1152. https://doi.org/10.3390/d15111152
Rahmouni C, Seifertová M, Šimková A (2023b) Revealing the hidden diversity of Gyrodactylus communities (Monogenea, Gyrodactylidae) from Nearctic Catostomidae and Leuciscidae fish hosts (Teleostei, Cypriniformes), with descriptions of ten new species. Parasite 30:40. https://doi.org/10.1051/parasite/2023035
Razo-Mendivil U, García-Vásquez A, Rubio-Godoy M (2016) Spot the difference: two cryptic species of Gyrodactylus von Nordmann, 1832 (Platyhelminthes: Monogenea) infecting Astyanax aeneus (Actinopterygii, Characidae) in Mexico. Parasitol Int 65:389–400. https://doi.org/10.1016/j.parint.2016.05.009
Reshetnikov AN, Sokolov SG, Protasova EN (2011) The host-specific parasite Nippotaenia mogurndae confirms introduction vectors of the fish Perccottus glenii in the Volga river basin. J Appl Ichthyol 27:1226–1231. https://doi.org/10.1111/j.1439-0426.2011.01792.x
Ribeiro F, Leunda PM (2012) Non-native fish impacts on Mediterranean freshwater ecosystems: current knowledge and research needs. Fisheries Manag Ecol 19:142–156. https://doi.org/10.1111/j.1365-2400.2011.00842.x
Roberts DW (2019) labdsv: Ordination and multivariate analysis for ecology. R package version 2.0–1
Roche B, Mattei J (1997) Les espèces animales introduites dans les eaux douces de Corse. Bull Fr Pêche Piscic. https://doi.org/10.1051/kmae:1997025
Roché B (2001) Atlas des poissons d’eau de douce de Corse. DIREN de CORSE. Bastia
Rolbiecki L, Ściążko M, Schütz J (2009) Parasitic fauna of the lake brown trout, Salmo trutta lacustris (Salmonidae), a little known endemic fish from Polish waters. Wiad Parazytol 55:445–450
Ronquist F, Teslenko M, van der Mark P et al (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542. https://doi.org/10.1093/sysbio/sys029
Roule L (1933) Le peuplement des cours d’eau de la Corse en poissons. Bull Fr Piscic 63:61–62. https://doi.org/10.1051/kmae:1933007
Rozas J, Ferrer-Mata A, Sánchez-DelBarrio JC et al (2017) DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol Biol Evol 34:3299–3302. https://doi.org/10.1093/molbev/msx248
Sáez-Gómez P, Prenda J (2019) Updating the distribution data of recently introduced freshwater fish in the Guadalquivir River Basin (Spain). BioInvasions Rec 8:924–932. https://doi.org/10.3391/bir.2019.8.4.21
Sándor D, Molnár K, Gibson D et al (2017) An investigation of the host-specificity of metacercariae of species of Apophallus (Digenea: Heterophyidae) in freshwater fishes using morphological, experimental and molecular methods. Parasitol Res 116:3065–3076. https://doi.org/10.1007/s00436-017-5617-5
Schönhuth S, Vukić J, Šanda R et al (2018) Phylogenetic relationships and classification of the Holarctic family Leuciscidae (Cypriniformes: Cyprinoidei). Mol Phylogenet Evol 127:781–799. https://doi.org/10.1016/j.ympev.2018.06.026
Shigoley MI, Rahmouni I, Louizi H et al (2023) First Study on Gyrodactylus (Monogenea: Gyrodactylidae) in Morocco, with description of a new species from Luciobarbus pallaryi and Luciobarbus ksibi (Actinopterygii: Cyprinidae). Animals 13:1624. https://doi.org/10.3390/ani13101624
Shukhgalter O, Chukalova N (2002) An investigation of “black spot” disease of bream (Abramis brama) from the Curonian Lagoon, south-eastern Baltic Sea. Bull Eur Assoc Fish Pathol 22:218–221
Šimková A, Řehulková E, Choudhury A, Seifertová M (2022) Host-specific parasites reveal the history and biogeographical contacts of their hosts: the Monogenea of Nearctic cyprinoid fishes. Biology 11:229. https://doi.org/10.3390/biology11020229
Tadese DA, Wubie A (2021) Impact of the introduction and domestication of alien fishes. Int Res J Eng Technol 08:8
Taraschewski H (2006) Hosts and parasites as aliens. J Helminthol 80:99–128. https://doi.org/10.1079/JOH2006364
Thalinger B, Oehm J, Mayr H et al (2016) Molecular prey identification in Central European piscivores. Mol Ecol Resour 16:123–137. https://doi.org/10.1111/1755-0998.12436
Tobler M, Schlupp I (2008) Influence of black spot disease on shoaling behaviour in female western mosquitofish, Gambusia affinis (Poeciliidae, Teleostei). Environ Biol Fishes 81:29–34. https://doi.org/10.1007/s10641-006-9153-x
Towns DR, Atkinson IAE, Daugherty CH (2006) Have the harmful effects of introduced rats on islands been exaggerated? Biol Invasions 8:863–891. https://doi.org/10.1007/s10530-005-0421-z
Turan D, Bayçelebi E, Özuluğ M et al (2023) Phoxinus abanticus, a new species from the Lake Abant drainage in Türkiye (Teleostei: Leuciscidae). J Fish Biol 102:1157–1167. https://doi.org/10.1111/jfb.15371
Tyutin A, Medyantseva E, Bazarov M, Tyutin V (2023) Distribution patterns of metacercariae of the Trematoda Apophallus muehlingi (Jagerskiold, 1899) in fingerlings in an invasive population of Clupeonella cultriventris (Nordmann, 1840) from the Gorky Reservoir (Upper Volga Basin). Russ J Biol Invasions 14:66–78. https://doi.org/10.1134/S2075111723010137
Vanhove MPM, Snoeks J, Volckaert F, AM Huyse T, (2011) First description of monogenean parasites in Lake Tanganyika: the cichlid Simochromis diagramma (Teleostei, Cichlidae) harbours a high diversity of Gyrodactylus species (Platyhelminthes, Monogenea). Parasitology 138:364–380. https://doi.org/10.1017/S0031182010001356
Vanhove MPM, Economou AN, Zogaris S et al (2014) The Gyrodactylus (Monogenea, Gyrodactylidae) parasite fauna of freshwater sand gobies (Teleostei, Gobioidei) in their centre of endemism, with description of seven new species. Parasitol Res 113:653–668. https://doi.org/10.1007/s00436-013-3693-8
Whiteman NK, Parker PG (2005) Using parasites to infer host population history: a new rationale for parasite conservation. Anim Conserv 8:175–181. https://doi.org/10.1017/S1367943005001915
Wickström LM, Haukisalmi V, Varis S et al (2003) Phylogeography of the circumpolar Paranoplocephala arctica species complex (Cestoda: Anoplocephalidae) parasitizing collared lemmings (Dicrostonyx spp.). Mol Ecol 12:3359–3371. https://doi.org/10.1046/j.1365-294X.2003.01985.x
Wilfert L, Jiggins FM (2014) Flies on the move: an inherited virus mirrors Drosophila melanogaster’s elusive ecology and demography. Mol Ecol 23:2093–2104. https://doi.org/10.1111/mec.12709
Williams H (1994) Parasitic worms of fish. CRC Press, London
Wisenden B, Martinez-Marquez J, Gracia E, McEwen D (2012) High intensity and prevalence of two species of trematode metacercariae in the fathead minnow (Pimephales promelas) with no compromise of minnow anti-predator competence. J Parasitol 98:722–727. https://doi.org/10.1645/GE-2454.1
Witkowski A, Grabowska J (2012) The non-indigenous freshwater fishes of Poland: threats for native ichthyofauna and consequence for fishery: a review. Acta Ichthyol Piscat 42:77–87. https://doi.org/10.3750/AIP2011.42.2.01
Wittrock DD, Bruce CS, Johnson AD (1991) Histochemistry and ultrastructure of the metacercarial cysts of blackspot trematodes Uvulifer ambloplitis and Neascus pyriformis. J Parasitol 77:454–460. https://doi.org/10.2307/3283135
Wu XY, Chilton NB, Zhu XQ et al (2005) Molecular and morphological evidence indicates that Pseudorhabdosynochus lantauensis (Monogenea: Diplectanidae) represents two species. Parasitology 130:669–677. https://doi.org/10.1017/S0031182004007152
Zangl L, Schäffer S, Daill D et al (2022) A comprehensive DNA barcode inventory of Austria’s fish species. PLoS ONE 17:e0268694. https://doi.org/10.1371/journal.pone.0268694
Zhang C, Zhao Y (2016) Species diversity and distribution of Inland Fishes in China. Science Press
Zhang W-R, Hao C-L, Arken K et al (2023) New species of Gyrodactylus von Nordmann, 1832 (Monogenoidea: Gyrodactylidae) from Gymnodiptychus dybowskii (Kessler, 1874) (Schizothoracinae) in the Kunes River (Yili River basin), China. Int J Parasitol Parasites Wildl 22:136–145. https://doi.org/10.1016/j.ijppaw.2023.10.002
Ziętara MS, Lumme J (2003) The crossroads of molecular, typological and biological species concepts: two new species of Gyrodactylus Nordmann, 1832 (Monogenea: Gyrodactylidae). Syst Parasitol 55:39–52. https://doi.org/10.1023/A:1023938415148
Ziętara MS, Lebedeva D, Muñoz G, Lumme J (2012) A monogenean fish parasite, Gyrodactylus chileani n. sp., belonging to a novel marine species lineage found in the South-Eastern Pacific and the Mediterranean and North Seas. Syst Parasitol 83:159–167. https://doi.org/10.1007/s11230-012-9379-2
Ziȩtara MS, Lumme J (2002) Speciation by host switch and adaptive radiation in a fish parasite genus Gyrodactylus (monogenea, Gyrodactylidae). Evolution 56:2445–2458. https://doi.org/10.1111/j.0014-3820.2002.tb00170.x
Zrnčić S, Oraić D, Mihaljević Ž et al (2009) First observation of Posthodiplostomum cuticola (Nordmann, 1832) metacercariae in cypriniformes from Croatia. Helminthologia 46:112–116. https://doi.org/10.2478/s11687-009-0022-y
Acknowledgements
The authors warmly thank the field agents of both the Fédération de la Corse pour la Pêche et la Protection du Milieu Aquatique: Paul-Jean Agostini, Alain Martin, Joseph Canale, and Olivier Saget, and of the Office Français de la Biodiversité, for their help with the sampling. Laboratory access and assistance was provided by the “Service de Systématique Moléculaire” of the Muséum National d’Histoire Naturelle (CNRS UMS 2700 2AD).
Funding
The present study was partially funded as a doctoral fellowship of the University of Corsica Pasquale Paoli and the Culletivittà di Corsica granted to Anaïs Esposito (no grant number available). This research is part of the GERHYCO interdisciplinary project dedicated to water management, ecology, and hydro-ecosystem services in an insular context, and it was financially supported by the Culletivittà di Corsica.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Anaïs Esposito, Gaël P.J. Denys, Vincent Haÿ, Quentin Godeaux, Joséphine Foata and Yann Quilichini. The first draft of the manuscript was written by Anaïs Esposito and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial or proprietary interests in any material discussed in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Esposito, A., Denys, G.P.J., Haÿ, V. et al. Multiple introduction pathways of non-native Phoxinus minnows (Teleostei: Leuciscidae) in Corsica revealed by its hidden diversity and their parasites. Biol Invasions (2024). https://doi.org/10.1007/s10530-024-03320-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10530-024-03320-7