Abstract
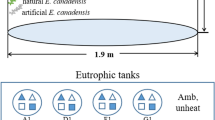
Knowledge of the factors controlling the growth of macrophytes is essential to forecasting their spatial distribution and management. Based on growth rates (µ), this study describes the cardinal temperatures of Egeria najas, and evaluates its growth potential. Cultures at 4 temperatures (15, 20, 25 and 30 °C) were monitored for 48 days. Plant growth was fitted to a logistic model to obtain growth rates (µ15: 0.02; µ20: 0.05; µ25: 0.10; µ30: 0.04 day−1) which were used for parameterization of the optimal temperature function, obtaining the cardinal temperatures (lower basal temperature: 5 °C; optimum temperature: 26.3 °C; upper basal temperature: 31.7 °C). Using the water temperature of the Óleo Lagoon (a tropical floodplain lagoon from which E. najas was harvested) and optimum temperature function, the seasonal variation of the E. najas µ was calculated. This procedure predicted that the highest µ are expected between September and April (rainy season); however, on average, the µ was higher between April and October (dry season). Although the temperature is of paramount importance for the growth of this species (Q10: 4.39), in aquatic environments with small thermal variations, turbidity and nutrient scarcity can decisively interfere with the growth of submerged macrophytes.


Similar content being viewed by others
Data availability
The four authors agree with the entire content (data and interpretation) of this manuscript and declare that the data are available under request.
References
Best, E. P. H., 1993. Models on metabolism of aquatic weeds and their application potential. In Pieterse, A. H. & K. J. Murphy (eds), Aquatic Weeds: The Ecology and Management of Nuisance Aquatic Vegetation Oxford University Press, Oxford: 254–273.
Bianchini Jr., I., A.L. Bitar & M.B. Cunha-Santino, 2006. Crescimento de Egeria najas Planchon da lagoa do Óleo em condições laboratoriais. In: Santos, J.E., J.S.R. Pires & L.E. Moschini (eds.). Estudos Integrados em Ecossistemas: Estação Ecológica de Jataí. vol. 4. EdUFSCar, São Carlos: 99–111.
Bianchini, I., Jr., M. B. Cunha-Santino, J. A. M. Milan, C. J. Rodrigues & J. H. P. Dias, 2010. Growth of Hydrilla verticillata (L.f) Royle under controlled conditions. Hydrobiologia 644(1): 301–312. https://doi.org/10.1007/s10750-010-0191-1.
Bianchini, I., Jr., M. B. Cunha-Santino, J. A. M. Milan, C. J. Rodrigues & J. H. P. Dias, 2015. Model parametrization for the growth of tree submerged aquatic macrophytes. Journal of Aquatic Plant Management 53: 64–73.
Bowie, G.L., W.B. Mills, D.B. Porcella, C.L. Campbell, J.R. Pagenkopf, G.L. Rupp, K.M. Johnson, P.W.H. Chan, S.A. Gherini & C.E. Chamberlin, 1985. Rates, constants, and kinetics formulations in surface water quality modeling. 2nd ed. U.S. Environmental Protection Agency, Athens (Georgia).
Camargo, A. F. M. & F. A. Esteves, 1995. Influence of water level variation on fertilization of an oxbow lake of Rio Mogi-Guaçu, state of São Paulo, Brazil. Hydrobiologia 299: 185–193. https://doi.org/10.1007/BF00767325.
Carr, G. M., H. C. Duthie & W. D. Taylor, 1997. Models of aquatic plant productivity: a review of the factors that influence growth. Aquatic Botany 59: 195–215. https://doi.org/10.1016/S0304-3770(97)00071-5.
Chapra, S. C. & R. P. Canale, 2010. Numerical Methods for Engineers, 6th ed. McGraw-Hill, New York:
Climatempo, 2022. Climatologia e histórico de previsão do tempo em Luíz Antônio, BR. https://www.climatempo.com.br/climatologia/2377/luizantonio-sp
Cook, C. D. K. & K. Urmi-Köning, 1984. A revision of the genus Egeria (Hydrocharitaceae). Aquatic Botany 19: 73–96. https://doi.org/10.1016/0304-3770(84)90009-3.
Cordeiro, P. F., F. F. Goulart, D. R. Macedo, M. C. S. Campos & S. R. Castro, 2020. Modeling of the potential distribution of Eichhornia crassipes on a global scale: risks and threats to water ecosystems. Revista Ambiente & Água 15(2): 2421. https://doi.org/10.4136/ambi-agua.2421.
Cunha-Santino, M. B., A. T. Fushita, A. C. Peret & I. Bianchini Jr., 2016. Morphometry and retention time as forcing functions to establishment and maintenance of aquatic macrophytes in a tropical reservoir. Brazilian Journal of Biology 76(3): 673–685. https://doi.org/10.1590/1519-6984.24214.
DAEE, 2022. Departamento de Águas e Energia Elétrica. Hidrologia. Banco de Dados Hidrológicos. http://www.hidrologia.daee.sp.gov.br/
Davidson, E. A. & I. A. Janssens, 2006. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440: 165–173. https://doi.org/10.1038/nature04514.
Duarte, C. M., D. Planas & J. Peñuelas, 1994. Macrophytes, taking control of ancestral home. In Margalef, R. (ed), Limnology Now: A Paradigm of Planetary Problems Elsevier, Amsterdam: 59–79.
Florêncio, F. M., D. C. Alves, F. M. Lansac-Tôha, M. J. Silveira & S. M. Thomaz, 2021. The success of the invasive macrophyte Hydrilla verticillata and its interactions with the native Egeria najas in response to environmental factors and plant abundance in a subtropical reservoir. Aquatic Botany 175: 103432. https://doi.org/10.1016/j.aquabot.2021.103432.
Fylstra, D., L. Lasdon, S. J. Watson & A. Waren, 1998. Design and use of the Microsoft Excel solver. Interfaces 28: 29–55. https://doi.org/10.1287/inte.28.5.29.
Glibert, P. M., 1998. Interactions of top-down and bottom-up control in planktonic nitrogen cycling. Hydrobiologia 363: 1–12. https://doi.org/10.1023/A:1003125805822.
Golterman, H.L., R.S. Clymo & M.A.M. Ohstand, 1978. Methods for Chemical Analysis of Fresh Waters. IBP Handbook nº 8. Blackwell, Oxford.
Gutiérrez, E.L., E.F. Ruiz, E.G. Uribe & J.M. Martínez, 2001. Biomass and productivity of water hyacinth and their application in control programs. In: Julien, M.H. & M.P. Hill (eds.), Biological and Integrated Control of Water Hyacinth, Eichhornia crassipes, T. D. Center and Ding Jianqing ACIAR Proceedings 102: 110–119.
Hachol, J., E. Bondar-Nowakowska & E. Nowakowska, 2019. Factors influencing macrophyte species richness in unmodified and altered watercourses. Polish Journal of Environmental Studies 28(2): 609–622. https://doi.org/10.15244/pjoes/85220.
Hamilton, S. K., O. C. Souza & M. E. Coutinho, 1998. Dynamics of floodplain inundation in the alluvial fan of the Taquari River (Pantanal, Brazil). Verhandlungen Des Internationalen Verein Limnologie 26: 916–922. https://doi.org/10.1080/03680770.1995.11900852.
He, Y., N. Song, H.-L. Jiang & H-L., 2018. Effects of dissolved organic matter leaching from macrophyte litter on black water events in shallow lakes. Environmental Science and Pollution Research 25: 9928–9939. https://doi.org/10.1007/s11356-018-1267-0.
Hudon, C., S. Lalonde & P. Gagnon, 2000. Ranking the effects of site exposure, plant growth form, water depth, and transparency on aquatic plant biomass. Canadian Journal of Fisheries and Aquatic Sciences 57(S1): 31–42. https://doi.org/10.1139/f99-232.
Hussner, A. & R. Lösch, 2005. Alien aquatic plants in a thermally abnormal river and their assembly to neophyte-dominated macrophyte stands (River Erft. Northrhine-Westphalia). Limnologica 35: 18–30. https://doi.org/10.1016/j.limno.2005.01.001.
Hogland, D.R. & D.I. Arnon, 1950. The water culture method of growing plants without soil. California Agricultural Experimental Station Circular 374. University of California, Berkeley.
IPCC, 2022. Summary for policymakers. In: Pörtner, H.-O., D.C. Roberts, E.S. Poloczanska, K. Mintenbeck, M. Tignor, A. Alegría, M. Craig, S. Langsdorf, S. Löschke, V. Möller & A. Okem (eds.), Climate Change 2022: Impacts, Adaptation and Vulnerability. Cambridge University Press, Cambridge: 3–33. (doi:https://doi.org/10.1017/9781009325844.001)
Janssen, A. B. G., S. Hilt, S. Kosten, J. J. M. Klein, H. W. Paerl & D. B. Van de Waal, 2021. Shifting states, shifting services: Linking regime shifts to changes in ecosystem services of shallow lakes. Freshwater Biology 66: 1–12. https://doi.org/10.1111/fwb.13582.
Jetter, K.M., J. Madsen, D. Bubenheim & J. Dong, 2021. Bioeconomic modeling of floating aquatic weeds in the Sacramento–San Joaquin River Delta. Journal of Aquatic Plant Management 59s: 98–106.
Jones, H. G., 2014. Plants and Microclimate: A Quantitative Approach to Environmental Plant Physiology, 3rd ed. Cambridge University Press, Cambridge:
Jørgensen, S. E. & B. D. Fath, 2010. Fundamentals of Ecological Modelling. Application in Environmental Management and Research, 4th ed. Elsevier, Amsterdam:
Kovalenko, K.E., F.M. Pelicice, L.B. Kats, J. Kotta & S.M. Thomaz, 2021. Aquatic invasive species: introduction to the Special Issue and dynamics of public interest. Hydrobiologia 848: 39–53. doi: https://doi.org/10.1007/s10750-021-04585-y
Kuar, M., M. Kumar, S. Sachdeva & S. K. Puri, 2018. Aquatic weeds as the next generation feedstock for sustainable bioenergy production. Bioresource Technology 251: 390–402. https://doi.org/10.1016/j.biortech.2017.11.082.
Lacoul, P. & B. Freedman, 2006. Environmental influences on aquatic plants in freshwater ecosystems. Environmental Reviews 14(2): 89–136. https://doi.org/10.1139/a06-001.
Langmuir, D., 1997. Aqueous Environmental Geochemistry, Prentice Hall, Upper Saddle River:
Lasaga, A. C., 1981. Transition state theory. In Lasaga, A. C. & R. J. Kirkpatrick (eds), Kinetics of Geochemical Processes Reviews in Mineralogy Mineralogy American Society, Washington DC: 1–68.
Machado, R., I. Bianchini Jr. & M. B. Cunha-Santino, 2020. Temperature and turbidity as drive forces to the growth of Egeria densa (Planchon) under to controlled conditions. Aquatic Botany 164: 103234. https://doi.org/10.1016/j.aquabot.2020.103234.
Madsen, J. D., 1998. Overview of the ecological assessment technology area. Journal of Aquatic Plant Management 36: 25–27.
Madsen, J.D. & C.M. Morgan, 2021. Water temperature controls the growth of waterhyacinth and South American sponge plant. Journal of Aquatic Plant Management 59s: 28–32.
Mackay, A. J., E. J. Muturi, M. P. Ward & B. Allan, 2016. Cascade of ecological consequences for West Nile virus transmission when aquatic macrophytes invade stormwater habitats. Ecological Applications 26(1): 219–232. https://doi.org/10.1890/15-0050.
Mahujchariyawong, J. & S. Ikeda, 2001. Modelling of environmental phytoremediation in eutrophic river - the case of water hyacinth harvest in Tha-chin River, Thailand. Ecological Modelling 142: 121–134. https://doi.org/10.1016/S0304-3800(01)00283-6.
Mitchell, D. S., 1974. Water weeds. In Mitchell, D. S. (ed), Aquatic vegetation and its use and control UNESCO, Paris: 13–22.
Neiff, J. J., 1999. El regimen de pulsos en ríos y grandes humedales de Sudamérica. In Malvarez, A. I. & P. Kandaus (eds), Tópicos sobre humedales subtropicales y templados de, Sudamerica Universidad de Buenos Aires, UNESCO, Montevideo: 97–146.
Neiff, J.J. & M. Neiff, 2022. Pulso, software para análisis de fenómenos recurrentes. Dir. Nac. Derecho de Autor No 236164 (Argentina), Buenos Aires. http//www.neiff.com.ar.
Nygaard, G., 1958. On the productivity of the bottom vegetation in Lake Grane Langsø. Verhandlungen Des Internationalen Verein Limnologie 18: 144–155. https://doi.org/10.1080/03680770.1956.11895394.
Passerini, M. D., M. B. Cunha-Santino & I. Bianchini Jr., 2016. Oxygen availability and temperature as driving forces for decomposition of aquatic macrophytes. Aquatic Botany 130: 1–10. https://doi.org/10.1016/j.aquabot.2015.12.003.
Petracco, P., 2006. Efeito das variáveis abióticas na produção primária de Egeria najas e Utricularia breviscapa da lagoa do Óleo (Estação Ecológica de Jataí, Luiz Antônio-SP). Tese, Universidade Federal de São Carlos, São Carlos. https://repositorio.ufscar.br/handle/ufscar/1564
Petracco, P., M. M. Pezzato & Cunha-Santino & I. Bianchini Jr., 2022. Net photosynthetic rates of Egeria najas and Utricularia breviscapa changes directed by seasonal hydrological variations. Brazilian Journal of Botany 45(3): 1129–1138. https://doi.org/10.1007/s40415-022-00828-x.
Pezzato, M. M., 2007. Macrófitas aquáticas submersas: fotossíntese, crescimento e variáveis abióticas da água. Tese, Universidade Federal de São Carlos, São Carlos. https://repositorio.ufscar.br/handle/ufscar/1603
Reitsema, R. E., P. Meire & J. Schoelynck, 2018. The future of freshwater macrophytes in a changing world: dissolved organic carbon quantity and quality and its interactions with macrophytes. Frontiers in Plant Science 9: 629. https://doi.org/10.3389/fpls.2018.00629.
Shelford, V. E., 1913. Animal Communities in Temperate America, University of Chicago Press, Chicago:
Shugart, H. H., R. A. Goldstein, R. V. O’Neill & J. B. Mankin, 1974. TEEM: a terrestrial ecosystem energy model for forests. Oecologica Plantarum 9(3): 231–264.
Silveira, M. J. & G. Thiébaut, 2017. Impact of climate warming on plant growth varied according to the season. Limnologica 65: 4–9. https://doi.org/10.1016/j.limno.2017.05.003.
Song, Y.-B., M.-Y. Zhou, Y.-L. Qin, J. H. C. Cornelissen & M. Dong, 2021. Nutrient effects on aquatic litter decomposition of free-floating plants are species dependent. Global Ecology and Conservation 30: 1748. https://doi.org/10.1016/j.gecco.2021.e01748.
Sousa, W. T. Z., S. M. Thomaz & K. J. Murphy, 2010. Response of native Egeria najas Planch. and invasive Hydrilla verticillata (L.f.) Royle to altered hydroecological regime in a subtropical river. Aquatic Botany 92: 40–48. https://doi.org/10.1016/j.aquabot.2009.10.002.
Strange, E. F., P. Landi, J. M. Hill & J. A. Coetzee, 2019. Modeling top-down and bottom-up drivers of a regime shift in invasive aquatic plant stable states. Frontiers in Plant Science 10: 889. https://doi.org/10.3389/fpls.2019.00889.
Straškraba, M., 1999. Self-organization, direct and indirect effects. In Tundisi, J. G. & M. Straškraba (eds), Theoretical Reservoir Ecology and Its Applications Backhuys Publishers, Leiden: 29–51.
Verhofstad, M. J. J. M. & E. S. Bakker, 2019. Classifying nuisance submerged vegetation depending on ecosystem services. Limnology 20: 55–68. https://doi.org/10.1007/s10201-017-0525-z.
Wallenstein, M. D. & M. N. Weintraub, 2008. Emerging tools for measuring and modeling the in situ activity of soil extracellular enzymes. Soil Biology and Biochemistry 40: 2098–2106. https://doi.org/10.1016/j.soilbio.2008.01.024.
Weber, M. A., L. A. Wainger, N. E. Harms & G. M. Nesslage, 2021. The economic value of research in managing invasive hydrilla in Florida public lakes. Lake and Reservoir Management 37(1): 63–76. https://doi.org/10.1080/10402381.2020.1824047.
Wetzel, R. G., 1964. A comparative study of the primary productivity of higher aquatic plants - periphyton and phytoplankton in a large shallow lake. Internationale Revue Der Gesamten Hydrobiologie Und Hydrographie 49: 1–61. https://doi.org/10.1002/iroh.19640490102.
Wu, H., B. Hao, H. Jo & Y. Cai, 2021. Seasonality and species specificity of submerged macrophyte biomass in shallow lakes under the influence of climate warming and eutrophication. Frontiers in Plant Science 12: 678259. (doi: https://doi.org/10.3389/fpls.2021.678259)
Yarrow, M., V. H. Marín, M. Finlayson, A. Tironi, L. E. Delgado & F. Fisher, 2009. The ecology of Egeria densa Planchon (Liliopsida: Alismatales): a wetland ecosystem engineer? Revista Chilena de Historia Natural 82: 299–313. https://doi.org/10.4067/S0716-078X2009000200010.
Yoshida, L. L., L. S. A. Valletta, M. B. Cunha-Santino & I. Bianchini Jr., 2022. A proposal for the equivalence between the rates of net photosynthesis and growth rate constants for submerged aquatic plants. Hydrobiologia 849: 77–88. https://doi.org/10.1007/s10750-021-04711-w.
Zhang, P., A. Kuramae, C. H. A. Van Leeuwen, M. Velthuis, E. Van Donk, J. Xu & E. S. Bakker, 2020. Interactive effects of rising temperature and nutrient enrichment on aquatic plant growth, stoichiometry, and palatability. Frontiers in Plant Science 11: 58. https://doi.org/10.3389/fpls.2020.00058.
Acknowledgements
The authors thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the scholarships (CNPq proc. nº 140406/2003-4; 306564/2020-0; CT-Hidro/CNPq, proc. nº 550188/2002-9; 140406/2003-4; Edital MCT/CNPq 02/2006—Universal; proc. nº 470527/2006-4).
Funding
This study was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq proc. nº 140406/2003-4; 306564/2020-0; CT-Hidro/CNPq, proc. nº 550188/2002-9; 140406/2003-4; Edital MCT/CNPq 02/2006-Universal; proc. nº 470527/2006-4).
Author information
Authors and Affiliations
Contributions
IBJ and MBCS developed the math procedures. MMP, PP and MBCS participated in the design of the study, field surveys, experimental works, data analyses, and wrote the manuscript. MBCS and IBJ participated in the design of study, edited the manuscript, and secured the funding. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest or competing interests.
Additional information
Handling editor: Andre Andrian Padial
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pezzato, M.M., Petracco, P., Cunha-Santino, M.B. et al. Assessment of cardinal temperatures of Egeria najas Planchon and its potential growth in a tropical floodplain lagoon. Hydrobiologia 850, 2127–2138 (2023). https://doi.org/10.1007/s10750-023-05224-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05224-4