Abstract
Nicidion Kinberg, 1865, and Paramarphysa Ehlers, 1887, were previously recognized as abranchiate groups of Eunice Cuvier, 1817, and Marphysa de Quatrefages, 1865, respectively. However, recent studies have demonstrated Nicidion is monophyletic, and the genus was redefined. Likewise, the character presence/absence of peristomial cirri, which traditionally was used to differentiate between Nicidion and Paramarphysa, is now considered non-diagnostic. Subsequently, the type species of Paramarphysa was recently transferred to Nicidion, implying that they are synonyms. Despite the above, some abranchiate species are still classified in Marphysa, raising the question of their positions within the genus. In the present study, we re-examined seven abranchiate species, studying their type material, and reviewing the literature, intending to disclose their taxonomic status. We concluded that only four of them belong to Nicidion: N. parvipes (Benham, 1927) n. comb., N. posteriobranchia (Day, 1962) n. comb., N. proppi (Averincev, 1972) n. comb., and N. saxicolas (Langerhans, 1881) n. comb. Two species, Marphysa simplex Langerhans, 1884, and Marphysa orientalis (Willey, 1905), were considered indeterminable. And one, ?Palola teres (Treadwell, 1922), is now considered incertae sedis because of insufficient information available. Additionally, we provided redescriptions of N. cincta Kinberg, 1865, type species of Nicidion, and N. hentscheli (Augener, 1931). Further character analysis on all Nicidion species suggested the genus consists of two groups (G1, G2) based on the distribution and presence and form of branchiae, the architecture of maxilla II, and the shape of the body’s posterior region. Also, the study of these and other characters such as the distribution of the swollen base of the ventral cirri and the coloration of the aciculae throughout the body, allow us to redefine the diagnosis of Nicidion.
Similar content being viewed by others
Introduction
The currently valid genus Nicidion Kinberg, 1865 (Annelida: Eunicidae), was previously recognized as an abranchiate group of Eunice Cuvier, 1817. The genus was proposed by Kinberg (1865) to contain those eunicid species with bilobed prostomium, peristomial cirri, and without branchiae. However, this last feature was regarded as a non-diagnostic character to separate genera (Orensanz 1990; Fauchald 1992a), triggering the synonymy of Nicidion with Eunice (Fauchald 1992a). A similar situation occurred to the genus Paramarphysa Ehlers, 1887, which was erected for the abranchiate species P. longula Ehlers, 1887 lacking peristomial cirri, but later considered a junior synonym of Marphysa de Quatrefages, 1865 (Carrera-Parra and Salazar-Vallejo 1998).
Nonetheless, in a recent phylogenetic study of Eunicidae, Nicidion was re-established based upon molecular and morphological evidence that supported the group’s monophyly. It was emended with species previously belonging to Eunice, Marphysa, and some others described initially in Nicidion (Zanol et al. 2014). This treatment indicated that the occurrence of peristomial cirri, a feature traditionally used to differentiate between those genera, was also not informative. Instead, the type of pectinate chaetae and the subacicular hook’s coloration were relevant to distinguish Nicidion from Marphysa (Zanol et al. 2014). Recently, Molina-Acevedo and Carrera-Parra (2017) also recognized Nicidion as valid based upon Zanol et al. (2014) and on some features with generic relevance discovered in the maxillary apparatus; likewise, they suggested that the absence of both the peristomial cirri and branchiae might be due to their young or early adult stage of development. Moreover, Molina-Acevedo and Carrera-Parra (2017) transferred two species previously described in Paramarphysa, including the type species, to Nicidion, suggesting the former as a junior synonym, but no further details on the synonymies were provided.
Despite the above, seven species of the “abranchiate” group are still recognized in Marphysa: Marphysa orientalis (Willey, 1905), M. parvipes (Benham, 1927), M. posteriobranchia Day, 1962, M. proppi (Averincev, 1972), M. saxicola Langerhans, 1881, M. simplex (Langerhans, 1884), and M. teres (Treadwell, 1922). Knowledge of these forgotten species is limited since they have not been studied since their original descriptions. Herein, we performed an exhaustive review of original literature and the type material of these species involved in this taxonomic problem. We provide redescriptions for four species and propose two species as indeterminable, and one as incertae sedis.
Material and methods
All type materials reviewed in this study are deposited at the following zoological collections and natural history museums: American Museum of Natural History, New York, USA (AMNH); the Natural History Museum, London, UK (BMNH); Natural History Museum, Vienna, Austria (NMHW); Swedish Museum of Natural History, Stockholm, Sweden (SMNH); Zoological Institute, Russian Academy of Sciences, Saint Petersburg, Russia (ZIN RAS); and Zoologisches Museum und Institute, Universität Hamburg, Hamburg, Germany (ZMH).
The redescriptions were made using the most recently proposed standards and terminologies of structures (Glasby and Hutchings 2010; Zanol et al. 2014; Molina-Acevedo and Carrera-Parra 2015, 2017; Molina-Acevedo 2018). Observations on the body were mostly performed with the stereomicroscope, although those made for the parapodia, chaetae, and occasionally the maxillary apparatus, were carried out with the compound microscope. The terminology of the maxillary apparatus proposed by Molina-Acevedo and Carrera-Parra (2017) was used. Paired and unpaired maxillae were indicated as “M” followed by a Roman number (e.g., MI, MIII). Five parapodia were dissected to describe and compare their morphology throughout the body: three parapodia from the anterior region (parapodia 1: between chaetigers 2 and 3, parapodia 2: between chaetigers 6 and 8, parapodia 3: between chaetigers 14 and 16), one from the middle region (complete specimen: parapodia in the middle of the body), and one from the posterior region (complete specimen: parapodia close to pygidium). Finally, the pectinate chaetae terminology follows Carrera-Parra (2009), Zanol et al. (2016), and Molina-Acevedo and Carrera-Parra (2017).
The type material was incomplete in most cases; the size of the organisms was thus standardized by measuring in millimeters (mm) the length from the anterior edge of prostomium to the end of chaetiger 10 (L10), the width of chaetiger 10 excluding parapodia (W10), and the total length of complete specimens (TL). A series of photographs of the diagnostic characters of specimens were taken, and Helicon Focus ® 6 (Method A) software was used to improve the depth of field. The final figures were assembled to plates using Adobe Photoshop®2020.
Results
Systematics
Order Eunicida Dales, 1962
Family Eunicidae Berthold, 1827
Nicidion Kinberg, 1865 restricted
Type species: Nicidion cincta Kinberg, 1865, by subsequent designation (Zanol et al. 2014)
Nicidion Kinberg, 1865: 564, 1910: 40, 42; –Ehlers 1868: 281, 304, 364; –Crossland 1904: 326; –Chamberlin 1919: 231; –Zanol et al. 2014: 95–96.
Paramarphysa Ehlers, 1887: 99 (new synonymy).
Diagnosis (after Molina-Acevedo and Carrera-Parra 2017). Prostomium slightly bilobed; five prostomial appendages arranged in horseshoe formation, palpostyles and ceratostyles with wrinkles or slightly marked grooves, without true articulations, exceeding length of prostomium; eyes present or absent (Fig. 1a). Peristomium with peristomial cirri (sometimes absent in juveniles or small adults), without articulations (Fig. 1a). Maxillary apparatus with four paired maxillae on both sides (MI, MII, MIV, MV) and one unpaired on the left side (MIII); elongated maxillary carriers with rectangular anterior region, triangular posterior end, with a pair of oval wings on lateral margins (Fig. 2b). MI forceps-like, lacking attachment lamella, with falcal arch not extended (Fig. 2b); base of MI with outer edges curved, inner edges distinctly curved and holding inner base of MII (Fig. 2b). MII without attachment lamella, cavity opening in both plates of similar size (Fig. 2b). MIII curved, forming part of distal arc, with rectangular or irregular-shaped attachment lamella situated at center of posterior edge of maxilla (Fig. 2b). MIV with sclerotized attachment lamella situated in anterior edge of maxilla (Fig. 2b). MV unidentate, without attachment lamella (Fig. 2b). Branchiae sometimes absent throughout (possibly only in juveniles or small adults), present only in a few anterior or posterior chaetigers (Fig. 1c, G1) or present from anterior-median chaetigers toward posterior end (sometimes absent in a few last chaetigers) (Fig. 1c, G2); branchiae filiform (Fig. 1c, G1), pectinate, or palmate with a short button-shaped branchial stem (Fig. 1c, G2); branchial filament digitiform (Fig. 1c, G1) or bluntly conical (Fig. 1c, G2). Dorsal cirri without articulations, longer than ventral cirri in anterior chaetigers, reduced in size gradually toward the posterior region (Fig. 1c). Ventral cirri short in first chaetigers, following one with an elongated, oval, swollen base with digitiform tip (Fig. 1c); swollen base present in less than half of body, ending shortly after the start of subacicular hook; in median to posterior region with ventral cirri short. Postchaetal lobe poorly developed. Supracicular chaetae only limbate and pectinate; thin and narrow heterodont pectinate chaetae in anterior chaetigers (Fig. 3j), and thin and wide heterodont pectinate chaetae in median and posterior chaetigers (Fig. 3k). Subacicular chaetae include only compound falcigers, bidentate. Subacicular hooks bidentate, reddish at the base or median or distal part of the hook, sometimes dark honey in posterior region. Aciculae blunt, sometimes mucronate (Fig. 3h), translucent in anteriormost chaetigers (Fig. 3d, e), dark in following chaetigers (Fig. 3f, g), aciculae change in color close to the start of subacicular hook. Pygidium with two pairs of anal cirri, without articulations.
Nicidion diagnostic characters. a anterior region of Nicidion posteriobranchia (Day, 1962) n. comb.; b parapodium 3 from Marphysa acicularum Webster, 1884 and Nicidion obtusa (Verrill, 1900); c posterior parapodium of Nicidion posteriobranchia (Day, 1962) n. comb. (G1) and N. angeli (Carrera-Parra and Salazar-Vallejo, 1998) (G2); d posterior region of Nicidion parvipes (Benham , 1927) (G1) and N. hentscheli (Augener, 1931) (G2). Parapodia of b in anterior view. DC, dorsal cirrus; PrL, prechaetal lobe; CL, chaetal lobe; PL, postchaetal lobe; VC, ventral cirrus; BBS, button-shaped branchial stem; A, acicula; SH, subacicular hook. Scale bars: a 0.65 mm; b Marphysa: 2 mm, Nicidion 0.1 mm; c G1 50 μm, G2 75 μm; d G1 0.6 mm, G2 1 mm
Maxillary apparatus architecture. a Marphysa leidii de Quatrefages, 1866; b Nicidion obtusa (Verrill, 1900); c left maxilla II from N. obtusa (G1) and Nicidion angeli (G2). AL, attachment lamella; MV, maxilla V; MIV, maxilla IV; MIII, maxilla III; CO, cavity opening; MII, maxilla II; MI, maxilla I; MC, maxillary carriers; ab, anterior border of the opening cavity. Arrows: in a pointing at the extended falcal arch; in b pointing at the curvature in basal inner edge of MI and holding inner base of MII; in c pointing at the teeth in MII exceeding the anterior border of the cavity opening or up to half of the maxilla. Scale bars: a 0.7 mm; b, c, 0.07 mm
Nicidion cincta Kinberg, 1865. Holotype SMNH-type-418. a anterior end, dorsal view; b anterior end, latero-ventral view; c body median-posterior region, ventral view; d parapodium 3; e parapodium 12; f parapodium 40; g parapodium 55; h mucronate aciculae, chaetiger 12; i aciculae from median region, chaetiger 40; j thin, narrow pectinate chaeta with short and slender teeth, chaetiger 12; k thin, wide heterodont pectinate chaeta with long and thick teeth, chaetiger 40; l compound falciger, chaetiger 12; m compound falciger, chaetiger 40; n subacicular hook, chaetiger 40. All parapodia in anterior view. Arrows: a pointing at peristomial cirri; b pointing at prostomium slightly bilobed; h pointing at the mucron; g upper pointing at aciculae, lower pointing at subacicular hook. Scale bars: a, b 0.9 mm; c 3.5 mm; d–g 0.2 mm; h, i, m, n 50 μm; j, k 12.5 μm; l 25 μm
Remarks. Kinberg (1865) established Nicidion within Eunicea (= Eunicidae) for members of the genus having a bilobed prostomium, nine maxillae: four paired and one unpaired, peristomial cirri, and lacking branchiae. Some authors considered Nicidion as valid at the genus level until the early 20th century (Ehlers 1868; Crossland 1904; Chamberlin 1919). However, Grube (1878) earlier considered Nicidion as part of Eunice by suspecting that the branchiae were not absent but present with a single filament only in a few most posterior chaetigers. Fauvel (1930: 3, 1932: 6) recognized Nicidion species as abranchiate Eunice but treated them at the subgenus level. Later, Hartman (1944, 1949) noted that Eunice (Nicidion) contained species smaller than proper Eunice and also emphasized the presence of branchiae in most posterior chaetigers of some species. Later, Orensanz (1990) regarded the presence/absence of branchiae as a character lacking value at the genus level. Based upon all the previous, Fauchald (1992a) considered Nicidion as a junior synonym of Eunice. Nonetheless, Zanol et al. (2014) established Nicidion as monophyletic and regarded it as a valid genus in the most recent phylogenetic reconstruction of Eunicidae.
The genus was initially proposed for three species: Nicidion longicirrata Kinberg, 1865 (Hawaii), N. cincta Kinberg, 1865 (Moorea Island), and N. gallapagensis Kinberg, 1865 (Galapagos). According to Hartman (1949), the first species was a junior synonym of Nicidion cariboea (previously Eunice cariboea Grube, 1856 from the Virgin Islands), and probably, for this reason, she assigned N. cariboea as type species of Nicidion (Art. 69.2.2 International Commission of Zoological Nomenclature (ICZN) 1999). In the same paper, Hartman (1949) redescribed N. cincta and considered N. gallapagensis as a species close to Palola Gray in Stair, 1847 rather than Nicidion, given the lack of subacicular hooks and the pectinate chaetae in type material, but she did not rule out that the absence of these features could be related to the missing posterior region in the type specimen. Later, Fauchald (1992a) determined that the synonymy between N. longicirrata and N. cariboea was incorrect due to morphological differences in prostomial appendices; however, Fauchald (1992a) considered to N. longicirrata related to Palola also for the absence of subacicular hooks and pectinate chaetae, but due to the poor condition of the type specimen he considered N. longicirrata as indeterminable species. From the three species described originally, just one had been treated as valid species, N. cincta. Because of the above, the subsequent designation of N. cariboea as type species of Nicidion made by Hartman (1949) is not adequate, since she selected a name not initially described in Nicidion as a type species. For this reason, under article 69.2.4 (ICZN 1999) and following recommendation 69A3 (ICZN 1999), the type designation of N. cincta as the type species of the genus made by Zanol et al. (2014) should be considered correct.
Most of the species currently valid in the genus were also described in either Eunice or Marphysa, although two other species were proposed in the now unaccepted genus Paramarphysa. Both P. longula (type species of Paramarphysa) and P. obtusa Verrill, 1900 were recently transferred from Marphysa to Nicidion (Molina-Acevedo and Carrera-Parra 2017). The genus Nicidion is currently made up of 14 species that show morphological patterns that allow us to recognize two major groupings. Group 1 (G1) is represented by species morphologically similar to N. cincta (type species) and consists of 11 species (Table 1). In G1, the branchiae may be present as a distinctly thick, single filament in a few most anterior or posterior chaetigers (Fig. 1c) (Carrera-Parra pers. obs.), or absent throughout; the body is generally of short size (approx. 100–200 chaetigers) with a sub-circular shape (in cross-section view) after the mandibular region, but tapering abruptly in the posteriormost chaetigers (Fig. 1d); the teeth in MII end more posterior than the anterior border of the cavity opening or up to half of the maxilla (Fig. 2c, G1); and finally, all the parapodia are supported by a thick acicula, sometimes up to twice as wide as the subacicular hook in median and posterior region (Fig. 1b (Nicidion); 1c G1). The branchiae in G1 species are poorly developed and challenging to distinguish from the dorsal cirri with the naked eye (Fig. 1c, G1; 8f), such as in N. amoureuxi (Rullier, 1974), N. insularis (Nogueira, Steiner & Amaral, 2001), N. obtusa (Verrill, 1900), and N. posteriobranchia (Day, 1962) n. comb. For some species in G1, the review of type materials would be necessary since a few features were not available in the literature.
On the other hand, group 2 (G2) (Table 2) comprises six species that have branchiae present between 50 and 65% of the chaetigers. The body of these species reaches a longer size (possibly more than 200 chaetigers); it has a hemispherical shape (in cross-section view) after the mandibular region but gradually tapering in the posteriormost chaetigers (Fig. 1d, G2), contrary to the G1. Also, branchiae are very well developed. They start from the very anterior region (<chaetiger 10) or near the chaetiger 20 or later but always end very close to the pygidial region. The branchiae consist of three to six elongated branchial filaments; almost twice longer than the dorsal cirrus (Fig. 1c, G2). Also, branchiae are pectinate, or palmate with a short button-shaped branchial stem (Fig. 1c, G2) Furthermore, in the MII, the species have teeth that end less posterior than the anterior border of the open cavity, or are located in less than half of the maxilla (Fig. 2c, G2). Finally, more than one acicula can be observed in the anterior region’s parapodia, but it is always reduced to one in the median-posterior region, sometimes up to twice as wide as the subacicular hook in the median and posterior region.
Nicidion was recently reinstated and established as monophyletic (Zanol et al. 2014). Nicidion species have been featured mainly by presenting irregular articulations in prostomial appendages (Zanol et al. 2014, 2020); however, this character braces ambiguity and unreliability. The definition of articulations in the prostomial appendages of Eunicidae has been controversial due to the lack of a clear distinction between wrinkles and true articulations (Zanol et al. 2007). There are no studies on the anatomy of these structures in eunicid species that support a distinctive innervation in each article, as demonstrated in other families such as Syllidae (Schmidbaur et al. 2020), particularly for Syllis Lamarck, 1818 species that have well-defined articulations on appendages. Each article in the antennae (as well as the tentacular and other cirri) is clearly separated by an internal septum rather than wrinkles (Schmidbaur et al. 2020: Fig. 10e), and they have a bundle of nerves on the distal edge innervated by a main longitudinal neurite bundle (Schmidbaur et al. 2020: Fig. 9e). These articulations of appendages have articles with a strongly marked constriction (Aguado and San Martín 2009), and hence, we judge them as true articulations. Zanol et al. (2007) defined a proper articulation in Eunicidae as one groove visible on all sides (continuous) of the style of the prostomial appendage. On the contrary, they considered that wrinkles or smooth styles are not articulations. Nevertheless, Zanol et al. (2014) extended the definition of articulation, stating that the articulations can be divided into two types: regular (i.e., true) and irregular. The irregular articulations were defined as wrinkled with at least some grooves distinct around the whole circumference of the style. Or even, if the styles present only wrinkles like in the N. cariboea, the appendages are considered to show irregular articulation. The above is confusing since Zanol et al. (2014) did not provide a proper delimitation of the articulations. Further, the wrinkles present in the prostomial appendages of eunicids should not be regarded as articulations since they are only barely noticeable, and fixation artifacts can form them. For instance, in some species of Marphysa, such as Marphysa fragilis Treadwell, 1911 and M. emiliae Molina-Acevedo and Carrera-Parra, 2017, these wrinkles, even surrounded by a coloration band, can be present in live specimens (Molina-Acevedo and Carrera-Parra 2017: pp. 15, Fig. 7), but disappear after fixation (Molina-Acevedo and Carrera-Parra 2017: pp. 20, Fig. 9). As for some other polychaete families, we consider that a strongly marked constriction present in the appendages of eunicids should only be considered as a true articulation, such as in some Leodice Lamarck, 1818 and Eunice species. Additional studies regarding the nervous and circulatory systems in the prostomial appendages can aid in restricting the definition of the articulations in eunicids.
The two major groups (G1, G2) are not monophyletic according to the last Eunicidae phylogeny; however, the relevant character and the morphological patterns herein studied could indicate that Nicidion is not a stable genus as previously considered. Some of these characters have been previously used to differentiate and establish new genera, e.g., the distribution of branchiae in Paucibranchia Molina-Acevedo, 2018, or the maxillary apparatus’s architecture in Treadwellphysa Molina-Acevedo and Carrera-Parra, 2017. Nevertheless, we strongly suggest a more in-depth review of the remaining species’ type materials focusing on the main distinguishing characters. Also, based on the review, a new phylogenetic analysis could be performed to shed light on whether the two groups actually belong to Nicidion or another genus.
Group 1
Nicidion cincta Kinberg, 1865
Nicidion cincta Kinberg, 1865: 564, 1910: 43, Pl. XVI, Fig. 21B–C, E–G; –Zanol et al. 2014: 95–96.
Eunice (Nicidion) cincta: –Hartman 1949: 80, Pl. XI, Fig. 10–12.
Eunice cincta: –Fauchald 1992a: 103, Fig. 30f–h, Tables 33 and 40.
Material examined. Holotype SMNH-type-418; French Polynesia, Moorea (= Eimeo); 17°20′ S, 149°48′ W; among corals; Eugenie Exp. 1851–53.
Description. Holotype incomplete, broken into two parts (first one with nine, second with 45 chaetigers), with 54 chaetigers, L10 = 3.5 mm, W10 = 1.4 mm (Fig. 3a–c). Anterior region damaged by a side cut (Fig. 3b), body with flat ventrum, of similar width throughout the body fragments. Last posterior part of second fragment was damaged (Fig. 3c).
Prostomium slightly bilobed, 0.7 mm long, 0.8 mm wide; lobes anteriorly rounded; without median sulcus (Fig. 3a), deep ventrally (Fig. 3b). Prostomial appendages in horseshoe arrangement, median antenna isolated by a gap. Palps reaching second peristomial ring; lateral and median antennae reaching first chaetiger (Fig. 3a). Palpophores and ceratophores ring-shaped, short, slender; palpostyles and ceratostyles digitiform, with some light wrinkles. Eyes as scars, between palps and lateral antennae.
Peristomium (0.7 mm long, 1.2 mm wide) wider than prostomium, first ring two times longer than second ring; separation between rings distinct on all sides (Fig. 3a–b). Peristomial cirri digitiform, short, reaching the posterior part of first peristomial ring, without articulation (Fig. 3a).
Maxillary apparatus lost, only illustration available according to Kinberg (1910).
Branchiae not observed.
First two parapodia smallest, best developed in chaetigers 1–17, following ones becoming gradually smaller. Dorsal cirri digitiform and longer than ventral cirri in all parapodia; best developed in chaetigers 3–14, following ones gradually decreasing in size (Fig. 3d–g). Prechaetal lobe short, as transverse fold in first 25 chaetigers; following one inconspicuous (Fig. 3d–g). Chaetal lobes rounded in first 14 chaetigers, shorter postchaetal lobes, aciculae emerging dorsally to midline; triangular from chaetiger 15, longer than other lobes, acicula emerging dorsally to midline (Fig. 3d–g). Postchaetal lobes poorly developed in first 24 chaetigers, rounded; inconspicuous in following chaetigers (Fig. 3d–g). Ventral cirri digitiform in first six chaetigers; from chaetigers 7 to 36 with an elongated oval swollen base and digitiform tip; from chaetiger 37 digitiform, gradually reducing in size (Fig. 3d–g).
Aciculae mucronate in first chaetigers (Fig. 3h), blunt in following ones; translucent in first 19 chaetigers, from chaetiger 20 basally reddish and amber distally (Fig. 3d–g, i). One per parapodium; aciculae two times wider than subacicular hook in median-posterior chaetigers (Fig. 3f, g).
Limbate chaetae of two lengths in the same parapodium, dorsalmost chaetae longer than ventral. Most pectinate chaetae broken and in bad condition; in anterior chaetigers: thin, narrow heterodont with short and slender teeth, with 1–2 pectinate chaetae per parapodium, with up to 11–12 teeth (Fig. 3j). In median and posterior chaetigers, thin, wide heterodont with long and wide teeth, up to 10–12 teeth (Fig. 3k). Compound falcigers bidentate in all parapodia; in anterior one’s with blades of similar length (41.3 μm, Fig. 3l); all with blunt teeth, of similar size; proximal tooth directed laterally, distal tooth directed upward. In last parapodium of the second fragment, falcigers with triangular teeth, distal tooth shorter than proximal (37.5 μm, Fig. 3m). Subacicular hooks broken or in bad condition, bidentate, starting from chaetigers 24 of second fragment, always one per parapodium; anterior hooks translucent basally and reddish distally, posterior hooks dark honey; most of the hooks with distal rounded tooth, smaller than proximal, directed upward; proximal tooth directed laterally (Fig. 3n).
Habitat. Skeletons of dead coral at 0.3–1.2-m depth (Kinberg 1865, 1910).
Distribution. Moorea Island, French Polynesia.
Remarks. Nicidion cincta was redescribed by Hartman (1949) and Fauchald (1992a). Herein we expand the description of the parapodia and the coloration of subacicular hook. Fauchald (1992a) commented about the lack of the swollen base of ventral cirri; however, a small, oval swollen base was found from chaetigers 7 to 36. Also, the reddish in subacicular hook seems reducing its intensity in the posterior region being dark honey.
Nicidion cincta closely resembles N. obtusa and N. proppi n. comb. by sharing mucronate aciculae in the anterior region. However, N. cincta has the ventral cirri digitiform in anteriormost chaetigers, whereas in both N. obtusa and N. proppi n. comb., they are sub-ovoid in the same parapodium. Also, the ventral cirri with swollen base in N. cincta (holotype, L10: 3.5 mm) are present in chaetigers 7 to 36, whereas in N. proppi n. comb. (holotype, L10: 1.1 mm), it is present in chaetigers 4 to 25, and in N. obtusa (additional material, L10:1.1–3.9 mm) from chaetigers 10 to 17–37. Finally, N. cincta has acicula two times wider than the subacicular hook throughout. In contrast, the aciculae and subacicular hook are of similar size throughout the body in both N. obtusa and N. proppi. The comparison of N. cincta with similar species is provided in Table 1.
Nicidion parvipes (Benham, 1927 ) n. comb.
Paramarphysa parvipes Benham, 1927: 89–90, Pl. 2, Figs. 35–41.
Material examined. Syntype BNHM 1928.2.29.54, slide BNHM.1933.3.831; BNHM.1933.3.8.33; BNHM.1933.3.8.32, stn 9. N.Z, seven miles E. of North Cape, Terra Nova, New Zealand, 03 Aug 1911, in sand and rock, 128-m depth.
Description. Syntype BNHM 1928.2.29.54 complete, with 96 chaetigers, L10 = 2.5 mm, W10 = 0.7 mm, LT = 18 mm. Anterior region with convex dorsum and flat ventrum; body depressed from chaetiger 8, widest at chaetiger 38, slightly tapering after chaetiger 52.
Prostomium slightly bilobed, 0.5 mm long, 0.6 mm wide; lobes anteriorly rounded; without median sulcus (Fig. 4a), deep ventrally (Fig. 4b). Prostomial appendages in horseshoe arrangement, median antenna isolated by a gap. Palps reaching middle of first peristomial ring; lateral antennae reaching second peristomial ring; median antenna broken. Palpophores and ceratophores ring-shaped, short, slender; palpostyles and ceratostyles digitiform, thick, with some light wrinkles. Eyes rounded, brown, between palps and lateral antennae.
Nicidion parvipes (Benham, 1927) n. comb. Syntype BNHM 1928.2.29.54. a anterior end, dorsal view; b anterior end, ventral view; c maxillary apparatus, dorsal view; d mandible; e posterior end, ventral view. Maxillary apparatus and mandible dyed with methyl green. Scale bars: a, b, e 0.6 mm; c 87.5 μm; d 0.1 mm
Peristomium (0.6 mm long, 0.8 mm wide) slightly larger than prostomium, first ring two times longer than second ring; separation between rings distinct on all sides (Fig. 4a, b). Ventral region smooth (Fig. 4b). Peristomial cirri not observed.
Maxillary apparatus with MF = 1 + 1, 3 + 4, 6 + 0, 2 + 7, 1 + 1 (Fig. 4c). MI 2.3 times longer than maxillary carriers. MI forcep-like, MI 7 times longer than closing system; ligament between MI and MII rectangular, sclerotized (Fig. 4c). MII wider than rest of maxillae, with triangular teeth; MII 3.6 times longer than cavity opening, last posterior tooth exceeds anterior border of the cavity opening (Fig. 4c); ligament between MII and MIII and right MIV slightly sclerotized. MIII with blunt teeth; with attachment lamella irregular, situated in the center of ventral edge of maxilla, sclerotized (Fig. 4c). Left MIV with teeth of similar size; attachment lamella semicircular, wide, better developed in central portion, situated along anterior edge of maxilla, sclerotized (Fig. 4c). Right MIV with blunt teeth; attachment lamella semicircular, wide, better developed in the central portion, situated along anterior edge of maxilla, sclerotized (Fig. 4c). MV square, with a short, rounded tooth. Mandibles amber; missing calcareous cutting plates, with poor sclerotized cutting plates (Fig. 4d).
Branchiae not observed.
First two parapodia smallest; best developed in chaetigers 3–20, following ones gradually smaller. Dorsal cirri digitiform in all parapodia; longer than ventral cirri in anterior chaetigers, shorter in median chaetigers, of similar size in posterior chaetigers; best developed in chaetigers 3–19, following ones gradually smaller (Fig. 5a–e). Prechaetal lobe as transverse fold in all parapodia (Fig. 5a–e). Chaetal lobes rounded in first 23 chaetigers, of similar size than other lobes, aciculae emerging in midline; triangular from chaetigers 24, longer than other lobes, aciculae emerging in midline (Fig. 5a–e). Postchaetal lobes poorly developed in first 17 chaetigers, rounded, progressively smaller from chaetiger 10; inconspicuous from chaetiger 18 (Fig. 5a–e). Ventral cirri ovoid-shaped in first chaetiger; from chaetigers 2 to 29 with elongated oval swollen base and digitiform tip; digitiform from chaetiger 30, gradually smaller (Fig. 5a–e).
Nicidion parvipes (Benham, 1927) n. comb. Syntype BNHM 1928.2.29.54. a parapodium 3; b parapodium 12; c parapodium 21; d parapodium 36; e parapodium 73; f thin, narrow heterodont pectinate chaeta with short and slender teeth, chaetiger 21; g thin, wide heterodont pectinate chaeta with long and slender teeth, chaetiger 73; h compound falciger, chaetiger 3; i subacicular hook, chaetiger 73. All parapodia in anterior view. Scale bars: a–e 30 μm; f–i 10 μm
Aciculae blunt; translucent in first 26 chaetigers, from chaetiger 27 basally reddish and translucent distally; one per parapodium (Fig. 5a–e). In posterior chaetigers aciculae of similar width than subacicular hook.
Limbate chaetae weakly winged; of two lengths in the same parapodium, dorsalmost chaetae longer than ventral. Two types of pectinate chaetae; in anterior chaetigers: thin, narrow heterodont with short and slender teeth, 1–2 pectinate chaetae per parapodium, up to eight teeth (Fig. 5f); in median-posterior chaetigers: thin, wide heterodont with long and slender teeth, 3–4 pectinate chaetae per parapodium, up to 15 teeth (Fig. 5g). Compound falcigers bidentate in all parapodia; in anterior chaetigers blades with similar length (18 μm Fig. 5h), all with blunt teeth, of similar size; proximal tooth directed laterally, distal tooth directed upward. In median-posterior chaetigers blades with similar length, shorter than anterior falcigers. Subacicular hooks bidentate, starting from chaetigers 26R–29L, one per parapodium; anterior-median hooks translucent-honey basally and reddish distally, posterior hooks dark honey; most of the hooks with triangular teeth, distal tooth smaller than proximal tooth, directed laterally; proximal tooth directed laterally (Fig. 5i).
Pygidium with dorsal pair of anal cirri as long as last chaetiger; ventral pair short, as long as pygidium (Fig. 4e).
Habitat. Sand and rocks at 128-m depth (Benham 1927)
Distribution. Terra Nova, New Zealand.
Remarks. Benham (1927) described Paramarphysa parvipes based on two slender specimens and classified them as Paramarphysa due to the absence of branchiae. After Orensanz (1990) and Carrera-Parra and Salazar-Vallejo (1998) recognized Paramarphysa as a junior synonym of Marphysa, this species became part of the long list of species in the latter genus. However, the combination of Marphysa parvipes was not officially published. Herein, we propose a new combination, N. parvipes n. comb. due to the similarity with the morphological diagnosis of Nicidion including the maxillary apparatus, the coloration pattern of subacicular hooks and aciculae throughout the body, and the form and distribution of the ventral cirri with a swollen base.
Nicidion parvipes n. comb. has a similarity with N. obtusa, N. posteriobranchia n. comb., N. proppi n. comb., and N. cariboea (Grube, 1856) in having aciculae of similar width than subacicular hook in posterior chaetigers. However, N. parvipes n. comb. (holotype, L10: 2.5 mm) has 3 + 4 teeth in MII, while M. obtusa (additional material, L10: 1.1–3.9 mm) MII: 6 + 6 teeth, N. posteriobranchia n. comb. (holotype, L10: 2.7 mm) MII: 5 + 6 teeth, N. proppi n. comb. (holotype, L10: 1.1 mm) MII: 7 + 6 and N. cariboea (syntype, L10: 2.4 mm) MII: 5 + 5 teeth. Furthermore, N. obtusa and N. posteriobranchia n. com. have mucronate aciculae in the anterior region, but the mucronate character is absent in N. parvipes n. comb. Also, N. parvipes n. comb. has ovoid-shaped ventral cirri in the first chaetiger (before the swollen base), while in N. posteriobranchia and N. cariboea, the ventral cirri are digitiform in anterior chaetigers. Likewise, in N. parvipes n. comb. , the subacicular hook starts in chaetiger 26, but for N. cariboea, the hook emerges starting from chaetiger 34. Finally, in N. parvipes n. comb., the ventral cirri with swollen base start in the second chaetiger, while in N. obtusa and N. proppi n. comb., the swollen base starts in chaetigers 10 and 4, respectively. The comparison of N. parvipes n. comb. with similar species is provided in Table 1.
Nicidion posteriobranchia (Day, 1962 ) n. comb.
Marphysa posteriobranchia Day, 1962: 645, Fig. 4a–e.
Material examined. Holotype BNHM 1961.14.3, sta. 131 A, St. Michaels-on-Sea, Natal, Oct 1961, coll. D McGregor.
Description. Holotype BNHM 1961.14.3 complete, broken into three parts (first part with 19 chaetigers, second with 49, third with 65 chaetigers) with 133 chaetigers, L10 = 2.7 mm, W10 = 1 mm, LT = 36 mm (Fig. 6a, b, f). Ventrally dissected from peristomium to chaetiger 4 (Fig. 6b). Anterior region with convex dorsum and flat ventrum; body depressed from chaetiger 8, widest at chaetiger 10, slightly tapering after chaetiger 14.
Nicidion posteriobranchia (Day, 1962) n. comb. Holotype BNHM 1961.14.3. a anterior end, dorsal view; b anterior end, ventral view; c maxillary apparatus, dorsal view; d left MI-II-III-IVV; e mandible; f posterior end, ventral view. LMI-II, ligament between MI and MII; LMII-III, ligament between MII and MIII; CO, cavity opening; dotted line indicates the maximum height of the cavity opening; MIc, curvature in outer edge of MI. Scale bars: a, b, f 0.7 mm; c, d 0.3 mm; e 0.2 mm
Prostomium slightly bilobed, 0.8 mm long, 0.6 mm wide; lobes anteriorly rounded; without median sulcus (Fig. 6a), deep ventrally (Fig. 6b). Prostomial appendages in horseshoe arrangement, median antenna isolated by a gap. Palps reaching second peristomial ring; lateral antennae reaching first chaetiger; median antenna reaching third chaetiger. Palpophores and ceratophores ring-shaped, short, slender; palpostyles and ceratostyles digitiform, slender, with some light wrinkles and at least one ring in ceratostyles. Eyes rounded, reddish, between palps and lateral antennae.
Peristomium (0.6 mm long, 1.2 mm wide) wider than prostomium, first ring two times longer than second ring; separation between rings distinct on all sides (Fig. 6a–b). Ventral region with a slight central depression and a couple of shallow wrinkles (Fig. 6b). Peristomial cirri not observed.
Maxillary apparatus with MF = 1 + 1, 5 + 6, 7 + 0, 3 + 9, 1 + 1 (Fig. 6c–d). MI 2 times longer than maxillary carriers. MI forceps-like, MI 6 times longer than closing system; ligament between MI and MII rectangular, slightly sclerotized (Fig. 6c–d). MII wider than rest of maxillae, with triangular teeth; MII 3 times longer than cavity opening, last two posterior teeth exceeds anterior border of the cavity opening (Fig. 6c–d); ligament between MII and MIII and right MIV slightly sclerotized. MIII with blunt teeth; with attachment lamella irregular, situated in center of ventral edge of maxilla, sclerotized (Fig. 6c–d). Left MIV with anterior tooth smaller; attachment lamella semicircular, wide, better developed in central portion, situated along anterior edge of maxilla, sclerotized (Fig. 6c–d). Right MIV with blunt teeth; attachment lamella semicircular, wide, better developed in the central portion, situated along anterior edge of maxilla, sclerotized (Fig. 6c–d). MV square, with a short-rounded tooth. Mandibles amber; missing calcareous cutting plates, with sclerotized cutting plates amber, with 10 growth rings (Fig. 6e).
Branchiae with only one filament, from chaetigers 97R–101L to 133 (Figs. 6f and 7f). Branchial filaments longer than dorsal cirri except in first four branchiae.
Nicidion posteriobranchia (Day, 1962) n. comb. Holotype BNHM 1961.14.3. a parapodium 3; b parapodium 15; c parapodium 30; d parapodium 63; e parapodium 105; f parapodium 125; g thin, narrow heterodont pectinate chaeta with short and slender teeth, chaetiger 3; h thin, wide heterodont pectinate chaeta with long and slender teeth, chaetiger 63; i compound falciger, chaetiger 3; j compound falciger, chaetiger 125; k subacicular hook, chaetiger 63. All parapodia in anterior view. Scale bars: a–f 50 μm; g–k 10 μm
First two parapodia smallest; best developed in chaetigers 3–10, following ones becoming gradually smaller. Dorsal cirri digitiform in anterior and posterior parapodia, conical in median chaetigers; longer than ventral cirri in all parapodia; best developed in chaetigers 3–17, following ones gradually smaller (Fig. 7a–f). Prechaetal lobe as a transverse fold in all parapodia (Fig. 7a–f). Chaetal lobes rounded in first 55 chaetigers, of similar size than other lobes, aciculae emerging dorsally to midline; triangular from chaetiger 56, longer than other lobes, aciculae emerging close to midline (Fig. 7a–f). Postchaetal lobes poorly developed in first 32 chaetigers, rounded, progressively smaller from chaetiger 13; from chaetiger 33 inconspicuous (Fig. 7a–f). Ventral cirri digitiform in first 4 chaetigers; from chaetigers 5 to 32 with elongated oval swollen base and digitiform tip; digitiform from chaetiger 33, gradually smaller (Fig. 7a–f).
Aciculae blunt; translucent in first 29 chaetigers, amber from 30 to 35, from chaetiger 36 basally reddish and translucent distally; one per parapodium (Fig. 7a–f). In posterior chaetigers aciculae of similar width as subacicular hook.
Limbate chaetae weakly winged; two lengths in the same parapodium, dorsalmost chaetae longer than ventral. Two types of pectinate chaetae; in anterior chaetigers: thin, narrow heterodont with short and slender teeth, 1–2 pectinate chaetae per parapodium, up to 12 teeth (Fig. 7g); in median-posterior chaetigers: thin, wide heterodont with long and slender teeth, 3–4 pectinate chaetae per parapodium, with up to 20 teeth (Fig. 7h). Compound falcigers bidentate in all parapodia; in anterior chaetigers with blades of similar length (26.6 μm, Fig. 7i), all with triangular teeth, of similar size; proximal tooth directed laterally, distal tooth directed upward. In median-posterior chaetigers with blades of similar length per parapodium, and similar length than falcigers in anterior chaetigers (24.5 μm, Fig. 7j); all with triangular teeth, distal tooth shorter than proximal, distal tooth directed upward, proximal tooth directed laterally. Subacicular hooks bidentate, starting from chaetigers 28L–29R, one or two per parapodium, upper hook unidentate; reddish along the hook and translucent distally; most of the hooks with triangular teeth, distal tooth smaller than proximal tooth, directed upward; proximal tooth directed upward (Fig. 7k).
Pygidium with dorsal pair of anal cirri as long as last chaetiger; ventral pair short, as long as pygidium (Fig. 6f).
Habitat. Burrowing under the base of coral Anomastrea irregularis von Marenzeller, 1901 (Day 1962).
Distribution. Natal, South Africa.
Remarks. Day (1962) described the species Marphysa posteriobranchia based on one specimen with branchiae restricted to the last part of the body. Although Day recognized Paramarphysa’s similarity, he decided to classify it in Marphysa due to the presence of branchiae. Herein, we propose a new combination, Nicidion posteriobranchia n. comb., since the morphology of the species coincides with the diagnosis of Nicidion concerning the form of the maxillary apparatus, the form and distribution of the ventral cirri with swollen base, and the color of aciculae and the subacicular hook throughout the body.
Nicidion posteriobranchia n. comb. has similarities with N. parvipes n. comb., N. cariboea, N. fuscafasciata Treadwell, 1922, and N. insularis in having aciculae blunt throughout the body, and the width of subacicular hook similar with aciculae in the posterior region. However, N. posteriobranchia n. comb. (L10: 2.7 mm) has MII with 5 + 6 teeth, while N. parvipes n. comb. (L10: 2.5 mm) has 3 + 4 teeth in MII. Furthermore, N. posteriobranchia n. comb. has translucent aciculae up to chaetiger 35; while in N. parvipes n. comb., the aciculae are translucent until chaetiger 26, and in N. insularis (original description) up to chaetiger 25. On the other hand, N. posteriobranchia n. comb. has a developed postchaetal lobe in first 32 chaetigers, and the ventral cirri with a swollen base end up to chaetiger 32; while in N. cariboea (type material, L10:2.4 mm) the postchaetal lobe is developed only in first 27 chaetigers, and ventral cirri with swollen base are present up to chaetiger 37, in N. insularis the ventral cirri with swollen base are developed until chaetiger 39. Also, in N. posteriobranchia n. comb. the subacicular hook start in chaetiger 28, instead of N. cariboea chaetiger 34, and in N. fuscafasciata (original description, LT:10. 3 mm) in chaetiger 23. The comparison of N. posteriobranchia n. comb. with similar species is provided in Table 1.
Nicidion proppi (Averincev, 1972 ) n. comb.
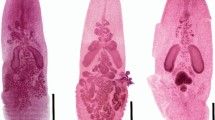
Nicidion proppi (Averincev, 1972) n. comb. Holotype ZISP-1/15809. a body, dorsal view; b anterior end, ventral view; c parapodium 7; d mucronate acicula, chaetiger 7; e thin, narrow pectinate chaeta with short and slender teeth, chaetiger 28, anterior view; f compound falciger, chaetiger 7; g subacicular hook, chaetiger 29. Scale bars: a–b 0.6 mm; c 20 μm; d–g 10 μm
Paramarphysa proppi Averincev, 1972: 173–174, Pl. 31, Figs. 1–10; –Orensanz 1990: 72.
Nicidion saxicola (Langerhans, 1881) n. comb. syntype NHM type 1884/IV/49. a anterior end, dorsal view; b maxillary apparatus, dorsal view; c thin, heterodont pectinate chaeta with long and slender teeth, chaetiger 87; d compound falciger, chaetiger 4; e compound falciger, chaetiger 87; f subacicular hook, chaetiger, 67. a and b stained with yellowish eosin; b the maxillary apparatus is observed through the skin, not dissected. Large arrow pointing at the maxillary carriers; short arrow pointing at the curvature in inner edge of MI. Scale bars: a 0.3 mm; b 0.1 mm; c–f 10 μm
Nicidion hentscheli (Augener, 1931) n. comb. Holotype ZMH V 10279. a anterior end, dorsal view; b anterior end, ventral view; c anterior end, lateral view; d maxillary apparatus, dorsal view; e mandible; f pygidium, dorsal view. Maxillary apparatus dyed with methyl green. Dark and thin lines in d indicate the forms in the MI of Nicidion; CO cavity opening in MII; dotted line indicates the maximum height of the cavity opening. Scale bars: a–c, f 1 mm; d 0.5 mm; e 0.4 mm
Material examined. Holotype ZIN RAS 1/15809, Kerguelen Island, 141 m, in broken bryozoans.
Description. Holotype ZISP-1/15809 incomplete, laterally dissected, posterior part in poor condition (Fig. 8a), with 37 chaetigers, L10 = 1.1 mm, W10 = 0.8 mm. Anterior region with dorsum convex and flat ventrum; body depressed from chaetiger 7, widest at chaetiger 18, with the same width throughout the body.
Prostomium slightly bilobed, 0.3 mm long, 0.3 mm wide; lobes anteriorly rounded; without median sulcus (Fig. 8a–b), deep ventrally. Prostomial appendages in horseshoe arrangement, median antenna isolated by a gap. Palps reaching middle of first peristomial ring; lateral antennae reaching second peristomial ring; median antennae reaching first chaetiger. Palpophores and ceratophores ring-shaped, short, slender; palpostyles and ceratostyles digitiform, wide, with some light wrinkles. Eyes reniform, brown, between palps and lateral antennae.
Peristomium (0.3 mm long, 0.6 mm wide) wider than prostomium, first ring two times longer than second ring; separation between rings distinct on all sides (Fig. 8a–b). Ventral region with a slight central depression, with a couple of shallow wrinkles (Fig. 8a). Peristomial cirri not observed.
Maxillary apparatus lost. According to the original description, FM = 1 + 1, 7 + 6, 6 + 0, 8 + 9.
Branchiae not observed.
First two parapodia smallest; best developed in chaetigers 1–26, following ones gradually smaller. Dorsal cirri digitiform and longer than ventral cirri in all parapodia; best developed in chaetigers 3–14, following ones gradually smaller. Prechaetal lobe as a transverse fold (Fig. 8c). Chaetal lobes rounded in first 26 chaetigers, of similar size than other lobes, aciculae emerging dorsally to midline; triangular from chaetiger 27, longer than other lobes, acicula emerging close to midline (Fig. 8c). Postchaetal lobes poorly developed in first 18 chaetigers, rounded; inconspicuous from chaetiger 19 (Fig. 8c). Ventral cirri ovoid-shaped in first three chaetigers; from chaetigers 4 to 25 with elongated oval swollen base and digitiform tip; digitiform from chaetiger 26, gradually smaller (Fig. 8c).
Aciculae mucronate in first chaetigers, blunt in following ones; translucent in first 16 chaetigers, from chaetiger 17 basally reddish and distally translucent; one per parapodium (Fig. 8d). In posterior chaetigers aciculae similar in width with subacicular hook.
Limbate chaetae weakly winged; of two length in the same parapodium, dorsalmost chaetae longer than ventral. Two types of pectinate chaetae; in anterior chaetigers: thin, narrow heterodont with short and slender teeth, 2–3 pectinate chaetae per parapodium, up to 14 teeth (Fig. 8e); in median-posterior chaetigers: thin, wide heterodont with long and slender teeth, 1–2 pectinate chaetae per parapodium, up to 12 teeth. Compound falcigers bidentate in all parapodia; with blades of similar length (17.5 μm, Fig. 8f) in each parapodia; in anterior chaetigers, all blades with triangular teeth, of similar size; proximal tooth directed laterally, distal tooth directed upward. In median chaetigers blades with triangular teeth, distal tooth shorter than proximal, directed upward, proximal tooth directed laterally. Subacicular hooks bidentate, starting from chaetiger 24, one hook per parapodium; most of the hooks translucent basally and reddish distally; most of the hooks with triangular teeth, distal tooth smaller than proximal tooth, directed upward; proximal tooth directed laterally (Fig. 8g).
Habitat. Within broken bryozoans at 141-m depth (Averincev 1972).
Distribution. Kerguelen Island, Southern Ocean.
Remarks. Marphysa proppi (Averincev, 1972) was considered indeterminable by Orensanz (1990) because it was presumably described based on a juvenile specimen. However, after reviewing the type material, we found that the specimen coincides with the diagnosis of Nicidion despite the absence of peristomial cirri and branchiae, characters that usually are absent in juveniles.
Nicidion proppi n. comb. is close to N. obtusa and N. cincta in the presence of mucronated aciculae in the anterior region. However, in N. proppi n. comb., the ventral cirri with swollen base start in chaetiger 4, instead of N. cincta and N. obtusa (type material, L10 3.1 mm) where the swollen base starts in chaetiger 7 and 10 respectively. Furthermore, N. proppi n. comb. has the ovoid-shaped anterior ventral cirri (before the swollen base), and aciculae have a similar width as the subacicular hook. In contrast, in N. cincta the ventral cirri are digitiform in anterior chaetigers (before the swollen base), and aciculae are two times wider than subacicular hook. On the other hand, in N. proppi n. comb. (L10: 1.1 mm) the subacicular hook start in chaetiger 24, and the ventral cirri’s swollen base is developed until chaetiger 25, while in N. obtusa (additional material, L10: 1.1 mm), the subacicular hook starts in chaetiger 18, and the swollen base is developed up to chaetiger 17. The comparison of N. proppi n. comb. with similar species is provided in Table 1.
Nicidion saxicola (Langerhans, 1881 ) n. comb.
Marphysa saxicola Langerhans, 1881: 111–112.
Material examined. Two syntypes mounted on slides, NHM type 1884/IV/49, Inv. Nr. 2379, Alt Inv. Nr. 3232, Tenerife, Canarias Island, Spain, Atlantic Ocean.
Description. Syntype NHM 1884/IV/49 incomplete, with 92 chaetigers, L10 = 1.2 mm, W10 = 0.7 mm, TL = 20 mm, last 27 chaetigers in regeneration. Anterior region with dorsum convex, flat venter; body depressed from chaetiger 13, widest at chaetiger 38, slightly tapering after chaetiger 61.
Prostomium slightly bilobed, 0.3 mm long, 0.3 mm wide; lobes frontally rounded; without median sulcus (Fig. 9a), deep ventrally. Prostomial appendages in horseshoe arrangement, median antenna isolated by a gap. Palps reaching second peristomial ring; lateral antennae reaching first chaetiger; lateral antennae reaching middle second chaetiger; median antennae reaching second chaetiger. Palpophores and ceratophores not observed; palpostyles and ceratostyles digitiform, slender, with some light wrinkles. Eyes triangular, brown, between palps and lateral antennae.
Peristomium (0.3 mm long, 0.7 mm wide) wider than prostomium, first ring two times longer than second ring, separation between rings distinct for all sides (Fig. 9a). In second syntype ventral region with a slight central depression and a couple of shallow wrinkles. Peristomial cirri not observed.
Maxillary apparatus with MF = 1 + 1, 4 + ?, 5 + 0, 3 + ?, 1 + 1 (Fig. 9b). MI 1.8 times longer than maxillary carriers. MI forceps-like, MI 8.3 times longer than closing system (Fig. 9b); ligament between MI and MII, sclerotized. MII wider than rest of maxillae; MII 3.1 times longer than cavity opening (Fig. 9b), last posterior tooth exceeds anterior border of the cavity opening; ligament between MII and MIII and right MIV, slightly sclerotized. MIII with attachment lamella irregular, situated in center of ventral edge of maxilla, sclerotized (Fig. 9b). Left MIV with attachment lamella triangular, wide, developed in basal portion. Right MIV with attachment lamella semicircular, wide, better developed in central portion (Fig. 9b). MV square. Mandibles not observed.
Branchiae not observed.
First two parapodia smallest; best developed in chaetigers 2–16, following ones gradually smaller. Dorsal cirri digitiform in anterior and posterior parapodia, form not observed in median parapodia, longer than ventral cirri in anterior and posterior parapodia. Prechaetal and chaetal lobe not observed. Postchaetal lobes poorly developed in first 13 chaetigers, rounded; inconspicuous from chaetiger 14. Ventral cirri digitiform in first five chaetigers; from chaetiger 6 to 25 with elongated oval swollen base and digitiform tip; conical from chaetiger 26.
Aciculae blunt; translucent in first 25 chaetigers, basally reddish and distally translucent from chaetiger 26; one per parapodium (Fig. 9c). From chaetiger 36 acicula two times wider than subacicular hook.
Limbate chaetae of two lengths in same parapodium, dorsalmost chaetae longer than ventral, reduced in number around chaetiger 30. Two types of pectinate chaetae; in anterior chaetigers: thin, narrow heterodont with short and slender teeth, 1–2 pectinate chaetae per parapodium, up to 12 teeth; in median-posterior chaetigers: thin, wide heterodont with long and slender teeth, with 2–3 pectinate per parapodium, and up to 12 teeth (Fig. 9c). Compound falcigers bidentate in all parapodia; in anterior region with blades of similar length (54 μm, Fig. 9d); with blunt teeth, of similar size, proximal tooth directed laterally, distal tooth directed upward. In median-posterior chaetigers blades of similar length (24 mm, Fig. 9e), shorter than anterior falcigers; with triangular teeth, distal tooth slightly shorter than proximal, tooth directed upward, proximal tooth directed laterally. Subacicular hooks bidentate, starting from chaetigers 26, one per parapodium; most of the hooks basally reddish and translucent distally; most of the hooks with triangular teeth, distal smaller than proximal, directed upward; proximal tooth directed laterally, distally directed upward (Fig. 9f).
Habitat. Dwelling in organic limestone layer of rocky beaches (Langerhans 1881).
Distribution. Tenerife, Canary Islands, Spain, Atlantic Ocean.
Remarks. Lavesque et al. (2017) commented that Marphysa saxicola was recently transferred to Nicidion by Arias and Núñez (2016); nevertheless, this information was obtained from an unpublished conference abstract; therefore, this nomenclatural act is not valid according to Article 8.2 ICZN (1999). Nonetheless, we agree with Arias and Núñez, and herein, we propose transfer Marphysa saxicola to Nicidion based on it possessing diagnosis features such as the coloration of the aciculae and subacicular hook through the body, and the form of the maxillary carrier and maxilla 1 (Fig. 9b).
Nicidion saxicola n. comb. resembles N. longula and N. cincta by having aciculae wider than subacicular hook in posterior chaetigers. However, N. saxicola n. comb. (L10: 1.2 mm) has the postchaetal lobe developed in first 13 chaetigers, and the ventral cirri with the swollen base end in chaetiger 25, instead of N. longula (type material, L10: 3.1 mm), the postchaetal lobe is developed in first 46 chaetigers, and ventral cirri are present until chaetiger 35; N. cincta has the postchaetal lobe developed in first 24 chaetigers, and the ventral cirri with the swollen base until chaetiger 36. Furthermore, N. saxicola n. comb. has the aciculae translucent in first 25 chaetigers, while in N. longula the translucent aciculae are present until chaetiger 35, and in N. cincta the translucent aciculae are in first 19 chaetigers. Additionally, in N. saxicola n. comb., the subacicular hook starts in chaetiger 26, while in N. longula the subacicular hook starts in chaetiger 33. Finally, N. saxicola n. comb. has the aciculae blunt in the anterior region, whereas in N. cincta, the aciculae are mucronate in anterior chaetigers. The comparison of N. proppi n. comb. with similar species is provided in Table 1.
Group 2
Nicidion hentscheli (Augener, 1931 )
Nicidion hentscheli (Augener, 1931) n. comb. Holotype ZMH V 10279. a parapodium 3; b parapodium 8; c parapodium 16; d parapodium 45; e parapodium 83; f parapodium 192; g thin, narrow heterodont pectinate chaeta with short and slender teeth, chaetiger 8; h thin, wide heterodont pectinate chaeta with long and slender teeth, chaetiger 120; i compound falciger, chaetiger 16; j compound falciger, chaetiger 120; k subacicular hook, chaetiger 83. All parapodia in anterior view. Arrow in figure d–e pointing at the button-shaped branchial stem. Scale bars: a–f 0.1 mm; j–k 10 μm
Marphysa hentscheli Augener, 1931: 20, Text Fig. 3a–d.
Nicidion hentscheli: –Zanol et al. 2014: 96.
Examined material: Holotype ZMH V-10279, Boa Viagem, Pernambuco, Brazil, 1925–1927.
Description. Holotype incomplete, with 198 chaetigers, L10 = 3.8 mm, W10 = 2.7 mm, TL = 71.2 mm. Anterior region with convex dorsum and flat ventrum; body depressed from chaetiger 12, widest at chaetiger 28, slightly tapering after chaetiger 76.
Prostomium slightly bilobed, 0.9 mm long, 1.1 mm wide; lobes anteriorly rounded; median sulcus barely visible (Fig. 10a), deep ventrally (Fig. 10b). Prostomial appendages in a horseshoe arrangement, median antenna isolated by a gap. Palps reaching middle of first peristomial ring; lateral antennae reaching first chaetiger; median antenna reaching second chaetiger. Palpophores and ceratophores ring-shaped, short, slender; palpostyles and ceratostyles digitiform, slender with some light wrinkles. Eyes not observed, only a possible scar on right side between palp and lateral antenna.
Peristomium (1.1 mm long, 1.8 mm wide) slightly larger and wider than prostomium, first ring two times longer than second ring; separation between rings distinct on all sides (Fig. 10a–c). Ventral region smooth. Peristomial cirri not observed.
Maxillary apparatus with MF = 1 + 1, 2 + 4, 4 + 0, 3 + 8, 1 + 1 (Fig. 10d). MI 2.2 times longer than maxillary carriers. MI forceps-like; MI 5 times longer than closing system; ligament between MI and MII, sclerotized. MII wider than rest of maxillae, with triangular teeth; MII 3.5 times longer than the cavity opening, posterior teeth reach anterior border of the cavity opening (Fig. 10d); ligament present between MII and MIII and right MIV sclerotized. MIII with blunt teeth; with attachment lamella not sclerotized (Fig. 10d). Left MIV with two anterior teeth longer; attachment lamella rectangular, wide, better developed in distal portion, situated 3/4 along anterior edge of maxilla, sclerotized (Fig. 10d). Right MIV with blunt teeth; attachment lamella semicircular, wide, better developed in the central portion, situated 1/2 along anterior edge of maxilla (Fig. 10d). MV square with a short, rounded tooth (Fig. 10d). Mandibles light brown; missing calcareous cutting plates and sclerotized cutting plates light brown, with 19 growth rings (Fig. 10e).
Branchiae palmate with a button-shaped branchial stem, with up to three long filaments, present from chaetiger 17 to last chaetiger of the fragment (Fig. 11d–f). One filament in chaetigers 17L–21L; two filaments in chaetigers 22L–31L; three filaments in chaetigers 32L–35L; two filaments in chaetigers 36L–86L; two or one filaments in chaetigers 87L–128L; three or two filaments from chaetigers 129L to last chaetiger of the fragment. Branchial filaments longer than dorsal cirri.
First three parapodia smallest; best developed in chaetigers 5–38, following ones gradually smaller. Dorsal cirri digitiform, and longer than ventral cirri in all parapodia; best developed in chaetigers 5–34, following ones gradually decreasing in size, smallest starting from chaetiger 61 (Fig. 11a–f). Prechaetal lobe as transverse folds in all parapodia (Fig. 11a–f). Chaetal lobes rounded in first 39 chaetigers, as large as postchaetal lobes, aciculae emerging dorsally to midline; triangular from chaetiger 40, longer than other lobes, aciculae emerging close to midline (Fig. 11a–f). Postchaetal lobes poorly developed in the first 49 chaetigers, rounded, inconspicuous from chaetiger 41 (Fig. 11a–f). Ventral cirri digitiform in first eight chaetigers; from chaetiger 9 to 51 with elongated oval swollen base and digitiform tip; digitiform from chaetiger 52, gradually smaller (Fig. 11a–f).
Aciculae blunt, translucent in first 39 chaetigers; from chaetiger 40 basally reddish and amber distally (Fig. 11a–f). Two aciculae per parapodium in the first 14 chaetigers, only one per parapodium from 15 towards the posterior end. In posterior chaetigers aciculae two times wider than subacicular hook.
Limbate chaetae weakly winged; of two lengths in the same parapodium, dorsalmost chaetae longer than ventral, reduced in number starting around chaetiger 25. Two types of pectinate chaetae; between chaetigers 5–35: thin, narrow heterodont with short and slender teeth, 1–2 pectinate chaetae per parapodium, up to 11 teeth (Fig. 11g); in median and posterior chaetigers: thin, wide heterodont with long and slender teeth, 2–3 pectinate chaetae per parapodium, up to 14 teeth (Fig. 11h). Compound falcigers bidentate in all parapodia; in anterior chaetigers with blade of two lengths (longer, 42 μm, Fig. 11i; shorter, 35 μm, Fig. 11j); all with blunt teeth, of similar size; proximal tooth directed laterally, distal tooth directed upward; shorter blade more abundant. In median-posterior chaetigers shorter blades of similar length (Fig. 11j), all with blunt teeth, distal tooth shorter than proximal, tooth directed upward, distal tooth directed laterally. Subacicular hooks bidentate, starting from chaetigers 41L–42R, always one per parapodium; anterior-median hooks translucent basally and reddish distally, posterior hooks dark honey; most of the hooks with distal tooth triangular, smaller than proximal, directed upward; proximal tooth directed laterally (Fig. 11k).
Pygidium broken, last chaetiger present near the posterior end of the specimen (Fig. 10f).
Habitat. Unknown.
Distribution. Boa Viagem, Pernambuco, Brazil, Atlantic Ocean.
Remarks. The species Marphysa hentscheli Augener, 1931 was transferred to Nicidion by Zanol et al. (2014) since molecular and morphological evidence showed the coincidence in diagnostic features with the genus Nicidion. Herein, a detailed redescription is included, in which we expand information about the shape of the body, prostomium, maxillary apparatus, branchiae, parapodia, and compound and simple chaetae.
Nicidion hentscheli and N. angeli Carrera-Parra & Salazar-Vallejo, 1998 have in common the palmate branchiae with a button-shaped branchial stem. However, N. hentscheli (holotype, L10 = 3.8 mm) has three branchial filaments, compound falcigers with blades of two lengths in anterior chaetigers, and aciculae two times wider than the subacicular hook in posterior chaetigers; instead, N. angeli (holotype, L10 = 3.8 mm) only has one branchial filament, compound falcigers with blades of a similar length in all chaetigers, and aciculae of similar width as subacicular hook in all chaetigers. The comparison of N. hentscheli with similar species is provided in Table 2.
Species incertae sedis
?Palola teres (Treadwell, 1922 )
Paramarphysa teres Treadwell, 1922: 153, Pl. 6, Figs. 2–6, Text Figs. 40 and 41.
Material examined. Holotype AMNH 1920-1538, Tutuila, Pago Pago Harbor, American Samoa, April–June 1920, coll. A. L. Treadwell.
Description. Holotype AMNH 1920-1538 incomplete, ventrally damaged from peristomium to chaetiger 2, with 204 chaetigers, L10 = 4.7 mm, W10 = 0.8 mm, LT = 71 mm. Anterior region with convex dorsum and flat ventrum; body depressed from chaetiger 4, widest at chaetiger 23, slightly tapering after chaetiger 67.
Prostomium slightly bilobed, 0.5 mm long, 0.6 mm wide; lobes anteriorly rounded; with light median sulcus (Fig. 12a), deep ventrally (Fig. 12b). Prostomial appendages in horseshoe arrangement, palps isolated by a gap. Palps reaching middle of first peristomial ring; lateral antennae reaching middle of second peristomial ring; median antenna broken reaching second peristomial ring. Palpophores and ceratophores ring-shaped, short, thick; palpostyles and ceratostyles digitiform, wide. Eyes rounded, brown, between palps and lateral antennae.
?Palola teres (Treadwell, 1922). Holotype AMNH 1920-1538. a anterior end, dorsal view; b anterior end, ventral view; c anterior end, lateral view; d parapodium 4; e parapodium 12; f parapodium 41; g parapodium 159; h compound falciger, chaetiger 12; i compound falciger, chaetiger 41. All parapodia in anterior view. Scale bars: a–c 1.2 mm; d–g 50 μm; h, i 10
Peristomium (0.8 mm long, 1 mm wide) larger than prostomium, first ring two times longer than second ring; separation between rings distinct in dorsal side (Fig. 12a, c). Ventral region dissected and not observed (Fig. 12b). Peristomial cirri not observed.
Maxillary apparatus lost. According to Treadwell, FM = 1 + 1, 3 + 3, ? + ?, ? + ?, ? + ?.
Branchiae not observed.
First two parapodia smallest, best developed in chaetigers 3–27, following ones becoming gradually smaller. Dorsal cirri digitiform and longer than ventral cirri in all parapodia; best developed in chaetigers 2–15, following ones gradually smaller (Fig. 12d–g). Prechaetal lobe as a transverse fold in all parapodia (Fig. 12d–g). Chaetal lobes rounded in first 36 chaetigers, of similar size than postchaetal lobe, aciculae emerging dorsally to midline; triangular from chaetigers 37, longer than other lobes, aciculae emerging slightly close to midline (Fig. 12d–g). Postchaetal lobes poorly developed in first 53 chaetigers, rounded, progressively smaller from chaetiger 14; inconspicuous from chaetiger 54 (Fig. 12d–g). Ventral cirri digitiform in first two chaetigers; from chaetigers 3 to 64 with elongated oval swollen base and digitiform tip; digitiform from chaetiger 30, gradually smaller (Fig. 12d–g).
Aciculae blunt; translucent in first 17 chaetigers, following ones basally reddish and translucent distally; one per parapodium (Fig. 12d–g).
Limbate chaetae weakly winged; of two lengths in the same parapodium, dorsalmost chaetae longer than ventral. Most of the chaetae broken. Pectinate chaetae absent. Compound falcigers bidentate in all parapodia; in anterior chaetigers with blades of similar length (28 μm, Fig. 12h); with triangular teeth, of similar size; proximal tooth directed laterally, distal tooth directed upward. In median chaetigers with blades of similar length per chaetiger, and similar length than blades in anterior chaetigers (24 μm, Fig. 12i); all with triangular teeth, distal tooth shorter than proximal, directed upward, proximal directed laterally. Subacicular hooks absent.
Habitat. Reef rocks (Treadwell 1922).
Distribution. Samoa, Pacific Ocean.
Remarks. Treadwell (1922) described and placed this species within Paramarphysa, probably due to the absence of peristomial cirri and branchiae. However, some other absences cast doubt on his identification. The specimens collected at Pago Pago Harbor did not have subacicular hooks, or pectinate chaetae, features that are present in Paramarphysa (= Nicidion). The absence of both characters is typical of Palola. Otherwise, although the maxillary apparatus was lost when the organism was described, the illustrations Pl. 6 Fig. 5 (Treadwell 1922) showed a short MI with a well-developed falcate arch, an elongated maxillary carrier without lateral wings, and a short MII with rounded teeth. This apparatus resembles the forms in Palola, or Lysidice Lamarck, 1818 that the same author describes in Plate 6. However, Treadwell drew flat mandibles with two thin, separate halves (Pl. 6 Fig. 6), which is common in Nicidion, Eunice, or Marphysa, yet very different from the typical spoon-shaped mandibles in Palola. The shape of the maxillary apparatus, absence of pectinates chaetae, and subacicular hook could indicate that the species belongs to Palola and not to Paramarphysa (= Nicidion). Moreover, the absence of peristomial cirri and branchiae would also indicate that it is a young specimen, and more material is necessary for appropriate identification.
Since it is not possible to identify to which genus the species belongs, herein, we consider it incertae sedis until more material from the region is studied, and its taxonomic status is clarified.
Discussion
Several species within Marphysa are long suspected of belonging to Nicidion. Herein, four Marphysa species were studied in detail and subsequently transferred to Nicidion by presenting the generic diagnostic features: Nicidion posteriobranchia n. comb., N. saxicola n. comb., N. parvipes n. comb., and N. proppi n. comb. Also, the type species of the genus N. cincta, as well as N. hentscheli are redescribed. Finally, one species was considered as incertae sedis as it is possibly a juvenile close to Palola. The redescription, transfer, or both, of the species treated in the present work allowed (1) to redefine Nicidion, (2) to confirm Paramarphysa as a junior synonym of Nicidion, (3) to increase the number of species recognized in Nicidion (17 species), and (4) to restrict even more the diagnosis of Marphysa to only branchiate species.
On the other hand, Amphiro simplex Langerhans, 1884 from Madeira, Portugal, and Marphysa orientalis Willey, 1905 from the Gulf of Mannar, India—both considered members of Marphysa nowadays—were described too briefly and the type materials were not found. Their descriptions suggest a similarity with Nicidion. Marphysa orientalis has only compound falcigers and translucent aciculae in the first 24 chaetigers, whereas A. simplex has branchiae with only one filament in the most of posterior parapodia. Nonetheless, both species are herein considered indeterminable because of the absence of type material and additional records and the short and insufficient original descriptions.
Based on this study, the diagnostic characters of Nicidion are as follow: swollen base of ventral cirri developed only in the anterior-median region (Fig. 1b, 7a–f), the base of MI without extended falcal arch, the inner edges of maxillae distinctly curved, holding the inner base of MII (Fig. 2b), and the maxillary carriers are elongated, bidentate subacicular hooks with dark color in median or distal region (Fig. 3f–g, n), and aciculae translucent in anteriormost chaetigers but dark in following chaetigers (Fig. 3d–g, h–i). Nicidion is clearly distinguished from Marphysa (sibling genera according to Zanol et al. 2014) because in the latter, the swollen base in ventral cirri is present in more than 50% of parapodia along the body. Also, the Marphysa species have the ventral cirri with swollen base rounded compared to the elongated oval shape in Nicidion (Fig. 1b). Furthermore, in Marphysa the MI presents a developed falcal arch, and the maxillary carriers are shorter (Fig. 2a–b) compared with Nicidion; and the aciculae in Marphysa are dark in all chaetigers.
Morphological studies that include taxonomic revision are imperative because it allows us to standardize the diagnostic characters. Subsequently, to visualize the patterns that bring together species with greater precision, whether in families, genera, or informal groups (e.g., G1, G2, or the groups in Marphysa Fauchald (1970)). In the case of the family Eunicidae, the patterns between genera have been unstable and continuously change over time with the advancement of morphological and molecular studies. For example, the number of prostomial appendages and the presence/absence of peristomial cirri were previously considered as relevant characters to distinguish genera, but, now, these characters are considered not informative for identification (Zanol et al. 2014). However, taxonomic revisions of Palola (Fauchald 1992b), Euniphysa Wesenberg-Lund, 1949 (Lu and Fauchald 2000), and Marphysa (Molina-Acevedo and Carrera-Parra 2017; Molina-Acevedo 2018) revealed morphological patterns that can provide greater stability to genera identification, such as architecture in the maxillary apparatus (Lu and Fauchald 2000; Molina-Acevedo and Carrera-Parra 2017; Molina-Acevedo 2018). Therefore, the present work reemphasizes further the importance of taxonomic revision and its relevance to improving classification in annelids.
References
Aguado MT, San Martín G (2009) Phylogeny of Syllidae (Polychaeta) based on morphological data. Zool Scr 38(4):379–402. https://doi.org/10.1111/j.1463-6409.2008.00380.x
Arias A, Núñez J (2016) Redescription and ontogeny of the controversial eunicid Marphysa saxicola Langerhans, 1881. 12th International Polychaete Conference, National Museum Wales, Cardiff, 1–5 August 2016
Augener H (1931) Die bodensässigen Polychäten nebst einer Hirudinee der Meteor-Fahrt. Mitteilungen der Zooologisches Staatinstitut und zoologisches Museum, Hamburg 44: 279–313
Averincev VG (1972) Benthic polychaetes Errantia from the Antarctic and Subantarctic collected by the Soviet Antarctic Expedition. Issled. fauny morei Zool. Inst. Akad. Nauk. USSR 11(19): 88–292
Benham WB (1927) Polychaeta [Terra Nova]. British Antarctic ‘Terra Nova’ Expedition Natural History Reports. Zoology 7(2):47–182
Berthold AA (1827) Latreille’s Natürliche Familien des Thierreichs: Aus dem Franzosischen, mit Anmerkungen und Zusätzen. Verlage Landes-Industrie-Comptoirs, Weimar, pp 606
Carrera-Parra LF (2009) Eunicidae Berthold, 1827. In: J.A. de León-González, J.R. Bastida-Zavala, L.F. Carrera-Parra, M.E. García-Garza, A. Peña-Rivera, S.I. Salazar-Vallejo y V. Solís-Weiss, Poliquetos (Annelida: Polychaeta) de México y América Tropical. Universidad Autónoma de Nuevo León, Monterrey, pp 165–181.
Carrera-Parra LF, Salazar-Vallejo SI (1998) A new genus and 12 new species of Eunicidae (Polychaeta) from the Caribbean Sea. J Mar Biol Assoc UK 78:145–182. https://doi.org/10.1017/S0025315400040005
Chamberlin RV (1919) The Annelida Polychaeta of the Albatross tropical pacific expeditions, 1891–1905. Mem Comp Zool Harv 48:1–514
Crossland C (1904) The marine fauna of Zanzibar and British East Africa, from collections made by Cyril Crossland in the years 1901 and 1902. - the Polychaeta. Part III. With which is incorporated the account of Stanley Gardiner’s collection made in the Maldive Archipelago in the year 1899. Proc Zool Soc London 74(1):287–330. https://doi.org/10.1111/j.1469-7998.1904.tb08292.x
Cuvier G (1817) Le régne animal distribué d’après son organisation, pour servir de base à l’histoire naturelle des animaux et d’introduction à l’anatomie comparée: Les Reptiles, les Poisson, les Mollusques et les Annélides. Deterville Libraire, Paris, pp 532
Dales RP (1962) The polychaete stomodeum and the inter-relationships of the families of Polychaeta. Proc Zool Soc London 139:389–428. https://doi.org/10.1111/j.1469-7998.1962.tb01837.x
Day JH (1962) Polychaeta from several localities in the western Indian Ocean. Proc Zool Soc London 139(4):627–656
de Quatrefages A (1865) Note sur la classification des Annélides. Cr Hebd Séanc Acad Sci 60:586–600
de Quatrefages A (1866 [1865]) Histoire Naturelle des Annelés Marins et d’Eau Douce: Annélides et Géphyriens. Tome Premier. Collection des Suites a Buffon formant avec les Oeuvres de cet auteur un Cours Complet d’Histoire Naturelle. Librairie Encyclopédique de Roret, Paris 588 pp. https://doi.org/10.5962/bhl.title.122818
Ehlers E (1868) Die Borstenwürmer (Annelida Chaetopoda) nach systematischen und anatomischen Untersuchungen dargestellt. Leipzig: Verlag von Wilhelm Engelmann, XX+269, pp. 269–748
Ehlers E (1887) Reports on the results of dredging, under the direction of L. F. Pourtalès, during the years 1868-1870, and of Alexander Agassiz, in the Gulf of Mexico (1877–78), and in the Caribbean Sea (1878–79), in the U.S. Coast Survey steamer “Blake”, Lieut-Com. C. D. Sigsbee, U.S.N. and Commander J. R. Bartlett, U.S.N., commanding. XXXI. Report on the Annelids. Mem. comp. Zool. Harv. 15(6): 1–335
Fauchald K (1970) Polychaetous annelids of the families Eunicidae, Lumbrineridae, Iphitimidae, Arabellidae, Lysaretidae and Dorvilleidae from Western Mexico. Allan Hancock Monogr. mar. Biol. 5: 1–335
Fauchald K (1992a) A review of the genus Eunice (Eunicidae: Polychaeta) based upon type material. Smithson Contrib Zool 523:1–422. https://doi.org/10.5479/si.00810282.523
Fauchald K (1992b) Review of the types of Palola (Eunicidae: Polychaeta). J Nat Hist 26:1177–1225
Fauvel P (1930) Supplement to the Littoral Fauna of Krusadai Island in the Gulf of Manaar. Part 1. Annelida Polychaeta of the Madras Government Museum. Bull. Madras Govt Mus. new. Ser. 1(2):1–72
Fauvel P (1932) Annelida Polychaeta of the Indian Museum, Calcutta. Indian Mus. Calcutta, Mem 12(1):1–262
Glasby CJ, Hutchings PA (2010) A new species of Marphysa Quatrefages, 1865 (Polychaeta: Eunicida: Eunicidae) from northern Australia and a review of similar taxa from the Indo-west Pacific, including the genus Nauphanta Kinberg, 1865. Zootaxa 2352:29–45. https://doi.org/10.11646/zootaxa.2352.1
Grube AE (1856) (pub. 1857). Annulata Örstediana. Enumeratio Annulatorum, quae in itinere per Indiam occidentalem et Americam centralem annis 1845-1848 suscepto legit cl. A.S. Örsted, adjectis speciebus nonnullis a cl. H. Kröyero in itinere ad Americam meridionalem collectis. Vidensk. Meddr. dansk naturh. Foren. i Köbenhavn 1856: 44–62
Grube AE (1878) Untersuchungen ueber die Familie Eunicea. Naturw. Schles. Gesells, Berlin, 1878: 37–62
Hartman O (1944) Polychaetous Annelids. Part V. Eunicea. Allan Hancock Pacif. Exped. 10(1): 1–237
Hartman O (1949) The marine annelids erected by Kinberg. With some notes on some other types in the Swedish State Museum. Ark Zool 42A(1):1–137
Hartmann-Schröder G (1965) Zur Kenntnis der eulitoralen Polychaetenfauna von Hawaii, Palmyra und Samoa. Abh. Verh. Naturwiss. Vereins Hamburg, Suppl. 9: 81–161
International Commission on Zoological Nomenclature [ICZN] (1999) International code of zoological nomenclature. 4th Edition. The International Trust for Zoological Nomenclature, London, pp 306
Kinberg JGH (1865) Annulata nova. Öfvers K VetenskAkad Förh Stockh 21:559–574
Kinberg JGH (1910) Annulater [Eugenies Resa]. Kongliga Svenska Fregatten Eugenies Resa omkring jorden under befal af C.A. Virgin aren 1851–1853. Vetenskapliga Iakttagelser pa Konung Oscar den Forstes befallningutgifna af K. Svenska Vetenskapsakademien. Zoologi I & VII. pp. 1–78, plates I to XXIX. Uppsala & Stockholm, Almquist and Wicksells Boktryckeri A/B
Lamarck JBPA (1818) Histoire naturelle des Animaux sans Vertèbres, préséntant les caractères généraux et particuliers de ces animaux, leur distribution, leurs classes, leurs familles, leurs genres, et la citation des principales espèces qui s’y rapportent; précédée d’une Introduction offrant la Détermination des caractères essentiels de l`Animal, sa distinction du végétal et des autres corps naturels, enfin, l’Exposition des Principes fondamentaux de la Zoologie, Paris, Déterville & Verdière Libraire, pp 612
Langerhans P (1881) Ueber einige canarische Anneliden. Nova Acta der Kaiserlichen Leopold-Carolin Deutschen Akademie der Naturforscher, Halle. 42(3): 95–124
Langerhans P (1884) Die Wurmfauna von Madeira. IV. Z. wiss. Zool. 40(2): 247–285
Lavesque N, Daffe G, Bonifácio P, Hutchings P (2017) A new species of the Marphysa sanguinea complex from French waters (Bay of Biscay, NE Atlantic) (Annelida, Eunicidae). ZooKeys 716:1–17. https://doi.org/10.3897/zookeys.716.14070
Lu H, Fauchald K (2000) A phylogenetic and biogeographic study of Euniphysa (Eunicidae, Polychaeta). J Nat Hist 34(7):997–1044
Molina-Acevedo IC (2018) Morphological revision of the subgroup 1 Fauchald, 1970 of Marphysa de Quatrefages, 1865 (Eunicidae: Polychaeta). Zootaxa 4480(1):001–125. https://doi.org/10.11646/zootaxa.4480.1.1
Molina-Acevedo IC, Carrera-Parra LF (2015) Reinstatement of three Grand Caribbean species of the Marphysa sanguinea complex (Polychaeta: Eunicidae). Zootaxa 3925:37–55. https://doi.org/10.11646/zootaxa.3925.1.3
Molina-Acevedo IC, Carrera-Parra LF (2017) Revision of Marphysa de Quatrefages, 1865 and some species of Nicidion Kinberg, 1865 with the erection of a new genus (Polychaeta: Eunicidae) from the Grand Caribbean. Zootaxa 4241:001–062. https://doi.org/10.11646/zootaxa.4241.1.1
Nogueira JMM, Steiner TM, Amaral ACZ (2001) Descriptions of two new species of Eunice Cuvier, 1817 (Polychaeta: Eunicidae) from coastal islands of the State of Sao Paulo, Brazil. Sci Mar 65(1):47–58
Orensanz JM (1990) The Eunicemorph polychaete annelids from Antarctic and Subantarctic Seas. With addenda to the Eunicemorpha of Argentina, Chile, New Zealand, Australia, and the Southern Indian Ocean. Antarctic Research Series 52: 1–183
Rullier F (1974) Quelques annelides polychetes de Cuba recueillies dans des eponges. Travaux du Muséum d’Histoire Naturelle “Grigore Antipa” 14: 9–77
Schmidbaur H, Schwaha T, Franzkoch R, Purschke G, Steiner G (2020) Within-family plasticity of nervous system architecture in Syllidae (Annelida, Errantia). Front Zool 17:20. https://doi.org/10.1186/s12983-020-00359-9
Stair JB (1847) An account of Palolo, a sea-worm eaten in the Navigator Islands, with a description by J.E. Gray. Proc Zool Soc London 15:17–18
Treadwell AL (1911) Polychaetous annelids from the Dry Tortugas, Florida. Bull Am Mus Nat Hist 30(1):1–12
Treadwell AL (1921) Leodicidae of the West Indian region. Carnegie Inst Wash Publ 15(293):1–131
Treadwell AL (1922) Leodicidae from Fiji and Samoa. Carnegie Inst Wash Publ 312:127–170
Verrill AE (1900) Additions to the Turbellaria, Nemertina, and Annelida of the Bermudas, with revisions of some New England genera and species. Trans Connecticut Acad Arts Sci 10(2):595–671. https://doi.org/10.5962/bhl.part.7035
von Marenzeller E (1901) Ostafrikanische Steinkorallen, gesammelt von Dr Stuhlmann 1888 und 1889. Mitt Natw Mus Hamburg 18:117–134
Webster HE (1884) Annelida from Bermuda collected by G. Brown Goode. Bull Am Mus Nat Hist 25:305–327
Wesenberg-Lund E (1949) Polychaetes of the Iranian Gulf. Danish Scientific Investigations in Iran. 4: 247–400
Willey A (1905) Report on the Polychaeta collected by Professor Herdman, at Ceylon, in 1902. Report to the Government of Ceylon on the Pearl Oyster Fisheries of the Gulf of Manaar by W.A. Herdman, with supplementary reports upon the Marine Biology of Ceylon, by Other Naturalists. Part IV. Supplementary Reports 30: 243–324
Zanol J, Fauchald K, Paiva P (2007) A phylogenetic analysis of the genus Eunice (Eunicidae, polychaete, Annelida). Zool J Linnean Soc 150:413–434
Zanol J, Halanych KM, Fauchald K (2014) Reconciling taxonomy and phylogeny in the bristleworm family Eunicidae (Polychaete: Annelida). Zool Scr 43:79–100. https://doi.org/10.1111/zsc.12034
Zanol J, da Silva TDSC, Hutchings PA (2016) Marphysa (Eunicidae, polychaete, Annelida) species of the sanguinea group from Australia, with comments on pseudo-cryptic species. Invertebr Biol 135:328–344. https://doi.org/10.1111/ivb.12146
Zanol J, Hutchings PA, Fauchald K (2020) Eunice sensu latu (Annelida: Eunicidae) from Australia: description of seven new species and comments on previously reported species of the genera Eunice, Leodice and Nicidion. Zootaxa 4748(1):001–043. https://doi.org/10.11646/zootaxa.4748.1.1
Acknowledgments
We sincerely thank Lily Berniker and Mark Siddall (AMNH), Emma Sherlock (BMNH), Helmut Sattmann (NHM), Lena Gustavsson (SMNH), Sergei Gagaev and Vladislav Potin (ZIN RAS), and Martin Schwentner, Kathrin Philipps-Bussau, and Petra Wagner (ZMH) for making available some of the materials that made this study possible; to Dr. Luis F. Carrera-Parra and Dr. Sergio I. Salazar-Vallejo for their advice and conversations about the Eunicidae family’s morphology, the time they took to review the thesis where this information comes from, and for the loan of the facilities in ECOSUR and devices to take photographs; to the University Malaysia Terengganu (UMT) for providing some facilities and access to publishing this manuscript; and to Biol. Humberto Bahena-Basave (ECOSUR, Mexico) for advice on digital photography and editing. We also thank Dieter Fiege and Lena Menzel (editors), Chris Glasby, and a second reviewer for the comments that contributed to the improvement of this final version.
Funding
During this research, the first author ICMA was supported by a scholarship from CONACyT (514117/298079) and DAAD Short-Term Grants (91673476).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
No animal testing was performed during this study.
Sampling and field studies
The study does not contain sampling material or data from field studies.
Data availability
All data generated or analyzed during this study are included in this published article.
Author contribution
The idea for the article, material preparation, data collection, literature search, and analysis was performed by ICMA. The first draft of the manuscript and comments on previous versions were written by all authors. All authors read and approved the final manuscript.
Additional information
Communicated by D. Fiege
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Molina-Acevedo, I.C., Idris, I. Revision of abranchiate species of Marphysa de Quatrefages, 1865 and their transference to Nicidion Kinberg, 1865 (Eunicidae: Annelida) with redescription of the type species N. cincta Kinberg, 1865. Mar. Biodivers. 51, 30 (2021). https://doi.org/10.1007/s12526-020-01157-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12526-020-01157-6