Orange Chromide, Pseudetroplus maculatus (Bloch., 1795): A Potential Euryhaline Fish Model to Evaluate Climate Change Adaptations in Fishes
- 1Mariculture Division, Karwar Regional Station of Indian Council of Agricultural Research (ICAR) -Central Marine Fisheries Research Institute, Karwar, India
- 2Mariculture Division, Calicut Regional Station of Indian Council of Agricultural Research (ICAR) - Central Marine Fisheries Research Institute, Calicut, India
- 3Marine Biotechnology Fish Nutrition and Health Division, Indian Council of Agricultural Research (ICAR), Central Marine Fisheries Research Institute, Kochi, India
Orange chromide, Pseudetroplus maculatus is a euryhaline species with both ornamental and food value. The species has several attributes similar to other fish model organisms such as smaller size, repeated breeding, ease of maintenance, and higher fecundity. A salinity tolerance study was performed in different salinities (0, 15, and 35 ppt) in triplicate introducing 10 fishes each (5.4 ± 0.08 g) in 12 plastic tanks of 60 L water-holding capacity. Fish were fed with commercial feed (1.2 mm and 40% protein) at 5% of body weight twice daily for 45 days. No significant variation (p< 0.05) in growth and survival was observed during the study indicating the wide salinity tolerance for the species. Experimental breeding of the species in freshwater and seawater (35 ppt) revealed the ability of the species to breed in varying salinities. Lenience in captive broodstock development, pair formation, and year-round natural breeding makes the seed production of the species easier. Characteristics such as multiple spawnings, a prolonged incubation period (48 to 72 hours) useful for elaborative embryonic studies, shorter larval development cycle (25 to 30 days), and better acceptance of live feed (Artemia nauplii and flakes) and commercial feed by the larvae make the species a potential euryhaline ornamental fish model to assess the physiological changes at different salinities. Minimal input requirements and lower capital and operational investments for the seed production of the species make it an ideal model organism for studying the impact of climatic and environmental changes on fish farming in different habitats.
Introduction
Modern biological research depends heavily on model organisms to reproduce and ensure the impact of research findings on a biological system. These ‘models’ help to evaluate complex biological processes related to human health or provide a powerful window into fundamental biological principles shared across the tree of life (Matthews and Vosshall, 2020). Model organisms have become reference points for an array of researchable issues for biological interventions (Ankeny and Leonelli, 2020). Put simply, model organisms are non-human species that are studied extensively in order to understand a range of biological phenomena. The US National Institutes of Health (NIH, 1999) listed the most important model organisms for biomedical research as mouse (Mus musculus), rat (Rattus norvegicus), zebrafish (Danio rerio), fruit fly (Drosophila melanogaster), nematode (Caenorhabditis elegans), baker’s yeast (Saccharomyces cerevisiae), and Thale cress (Arabidopsis thaliana).
Climate change has a direct impact on various habitats related to fishes. Major physical impacts of climate change on aquaculture are fluctuations in precipitation, salinity, temperature, dissolved oxygen level, pH, and nutrient concentration (Whitney et al., 2016). Variations in these parameters directly or indirectly affect the growth and physiology of fishes in all salinity regimes such as freshwater, brackishwater, and marine habitats. Sundell et al. (2021) reported metabolic changes in euryhaline fish due to reduced water salinity. Whitney et al. (2016) reviewed the impact of various climate change factors on the physiology of inland fishes from North America. But in order to attain a clear picture of the impact of climate alteration implications on all these salinity regimes a model organism that thrives well in all these habitats is the need of the hour.
Orange chromide, P. maculatus is a euryhaline fish with a wide distribution that can survive in fluctuating salinity. This species is endemic to brackish streams, lagoons, estuaries, and the lower reaches of rivers in peninsular India and Sri Lanka. The colour and body patterns of the species make it an important fish for the ornamental trade (Bindu and Padmakumar, 2012; Raghavan et al., 2013; Shilta et al., 2016). Some earlier studies (Pampapathi Rao, 1958; Virabhadrachari, 1961) reported the ability of this species to thrive in extreme salinities ranging from freshwater to salinities up to 100 ppt due to the structural changes in the gills, kidney, and intestine. It is reported that the species can have pair formation, mating, and breeding in different salinities ranging from freshwater (Bindu and Padmakumar, 2012) to marine conditions (Shilta et al., 2016)
The present report is an attempt to evaluate the merits of the ornamental fish P. maculatus as a potential candidate to become a model organism to evaluate the impact of climate change on fishes living in different salinity regimes.
Materials and Methods
Evaluation of the Characteristics of the Model Organism
Before initiating the breeding and salinity tolerance studies, the characteristics of the selected species as a model organism were evaluated by comparing the criteria to other two well-established fish models such as zebrafish (Daneo rerio) and Medaka (Oryzias latipes), using published data. Furthermore, the major objectives of the current study were highlighted in the list of important steps involved in the development of a new model organism as described by Matthews and Vosshall (2020).
Fish
Akalapuzha is a backwater connected to the Korapuzha estuary which flows through Calicut, Kerala, India that drains into the Arabian sea. Two hundred and fifty live fish of P. maculatus were caught from Akalapuzha (11° 49.498286 N and 75°75.670861 E) using a trap operated at 4 m depth and were brought to Calicut Regional Station of ICAR- Central Marine Fisheries Research Institute (CMFRI) for the purpose of the research.
Species Identification and Confirmation
For species identification based on conventional methods, counts and measurements were carried out according to Jayaram (2002); Jayaram (2010), and Mathews (2016). Morphometric measurements were accurately recorded using a digital vernier caliper (accuracy of 0.01 mm). Total length (TL- distance from the snout to the tip of the caudal fin), fork length (FL -distance from the snout to the end of the middle caudal fin rays), and standard length (SL distance from the snout to the posterior end of the last vertebra) were measured to the nearest mm using a measuring board and the total weight of the fish (W, wet weight) were recorded to the nearest 0.1 g using a digital analytical balance. In addition, various morphometric characters such as the proportion of Head length (HD), Body depth (BD), Snout length (SNL), Pre-dorsal length (PDL), Pre- pectoral length (PPL), Pre-ventral length (PVL), Pre-anal length (PAL) and Caudal depth (CD) to the standard length were calculated. Counts and measurements were compared to the data reported by Mathews (2016) for P. maculatus.
For molecular confirmation, five samples of each of the species in the study P. maculatus, and Pearlspot, Etroplus suratensis of its closely related genus Etroplus were collected and specific morphological characteristics were examined. Fin clip of each specimen was preserved in 90% ethanol for DNA extraction. Total genomic DNA was extracted from fin using DNeasy Blood and Tissue Kit (Valencia, CA) following the manufacturer’s protocol. A 650 bp region from the COI gene was amplified using the primer pair Fish F1 [5’-TCAACCAACCACAAAGACATTGGCAC-3’] and Fish R1 [5’-TAGACTTCTGGGTGGCCAAAGAATCA-3’] (Ward et al., 2005). PCR amplifications were performed in a volume of 25 μl containing 12.5 µl of TaKaRa EmeraldAmp GT PCR Master Mix, 0.5 µl of each primer (10mM), and 1 µl of 50 ng/µl DNA template. The PCR conditions consisted of initial denaturation at 95°C for 5 min; followed by 30 cycles of denaturation at 95°C for 30 s, annealing at 50°C for 30 s, and extension at 72°C for 45 s; a final extension step at 72°C for 10 min, and then held at 4°C. The PCR products were visualised in 1.5% agarose gel, purified, bi-directionally sequenced, and aligned in MEGA X (Kumar et al., 2018). Sequence similarity search was performed using the BLAST algorithm in GenBank and COI sequences of P. maculatus and members of its related genus Etroplus (Pearlspot, E. suratensis, and Canara pearlspot, E. canarensis) were mined from GenBank. The data was aligned with Polymixia japonica (AB034826) as an outgroup and phylogenetic tree was produced using MrBayes (GTR+G+I substitution model, 500,0000 chain length with 25% burn-in) (Huelsenbeck and Ronquist, 2001). Genetic divergence (uncorrected p-distance under uniform rates) was calculated using MEGA X (Kumar et al., 2018) with COI data of 606bp trimmed length. All sequences in this study were submitted to the NCBI database GenBank (https://www.ncbi.nlm.nih.gov/) under accession nos. OM992292-OM992296 and OM988400- OM988401.
Biology of the Species
The sex ratio of the moribund specimens collected from the wild was determined by dissecting the gonad.
The total length of the fish from the tip of the snout to the tip of the caudal fin was measured using a 1 m wooden scale with an accuracy of 1 mm. The total weight of the fish was measured using an electronic weighing balance (Sartorius, Germany) with an accuracy of 0.001 g. The length-weight relationship was calculated using the formula Log (WEIGHT) = a + [b x log (LENGTH)] where ‘a’ is the intercept and ‘b’ is the slope of the linear regression on the log-transformed weight (g) and length data (cm). The condition factor (k) of the fish in their habitat was determined using the equation as per Gomiero and Braga (2005). k = (W x 100)/L3 where k =condition factor; W=the weight of the fish in gram (g); L= the total length of the fish in centimetres (cm). The relative length of the gut of P. maculatus was measured to the nearest 0.1 cm as described by Euzen (1987) through the following equation: RLG= Length of gut/total body length.
For ova diameter studies ovarian biopsy samples were analysed using a stereo zoom microscope (Zeiss, China) with digital imaging facility (software: Zen 3.0, Germany) and a compound microscope (Zeiss, Germany) with digital imaging facility (ProgRes, Germany) for identifying the maturation stages based on the diameter and developmental stage of the oocytes present in the ovary.
Salinity Tolerance Study
The present study was conducted at the marine hatchery complex of Calicut Research Centre of ICAR-CMFRI to determine the salinity tolerance of P. maculatus to know the scope of this species as a marine ornamental fish. For the determination of salinity tolerance, the fish were reared in different salinities such as 0, 15, and 35 ppt respectively. The salinities were checked using a hand refractometer. Twelve plastic tanks of 60 L capacity were filled with water of varying salinities (50 L). All tanks were provided with uniform aeration. Ten numbers of P. maculatus were directly stocked in freshwater (0 ppt), brackish water (15 ppt), and seawater (35 ppt) and reared for 45 days. The study was carried out at room temperature (28.5C) under natural photoperiods. Fish were fed with commercial pellet feed (Nutrila 1.2 mm, Growel Feeds Pvt Ltd. India; 40% protein) at 5% of initial biomass. The fish were fed twice a day during daytime (8 am and 4 pm). At the end of the experiment, the number of fish in each tank was counted and the survival rate (%) was calculated by the following formula: Survival (%) = (Total number of fish present x 100)/Total number of fish stocked.
After 45 days, fish from each unit were weighed and sampled for tissue analysis after 24 h of fasting. Fish were anaesthetized with clove oil (50 µL L-1) and blood was collected from the caudal vein. Serum was collected by centrifuging the blood for 10 minutes at 0.5 g and stored at -20°C for subsequent analysis. For the collection of liver tissue, fish were euthanized and dissected. The liver tissue was weighed and collected in labelled tubes, added and homogenized with 0.25 00M sucrose buffer, and stored at -20°C until analysis. Triglycerides, cholesterol, total protein, albumin, globulin, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) levels in the liver and serum samples were measured using diagnostic kits (Tulip Diagnostics Ltd., Goa, India) according to the manufacturer’s instructions as per Ebeneezar et al. (2020).
Breeding in Different Salinities
About 150 count of adult fish (total length ranging from 5.2 to 8.7 cm, total weight ranging from 3.4 to 10.6 g) were stocked in two FRP tanks (1-ton capacity filled with 800 L chlorine-treated water) containing 0 and 35 ppt saline water with sufficient aeration. The fishes were fed with pellet feed (Nutrila 1.2 mm, Growel Feeds Pvt Ltd. India; 40% protein) at 2.5% of biomass. The fish were fed twice a day during daytime (8 am and 4 pm). Daily water exchange (20% of total volume) and tank bottom cleaning was performed. Two weeks after stocking the fish started pair formation. The fish that formed breeding pairs were directly stocked into plastic tanks (60 L breeding tanks) filled with 0 and 35 ppt water (50 L) with replications. No recirculating system was provided for the breeding tanks. The fish were fed with commercial pellet feed (Nutrila 1.2 mm, Growel Feeds Pvt Ltd.) containing 40% protein at 5% of biomass till spawning. The pellet feeds were given twice daily during daytime (8 am and 4 pm) at 2.5% of biomass for each of the two feeding times.
The breeding tanks were not provided with separate enclosures for brooders since the fish shows a high degree of parental care. Since P. maculatus is a substrate spawner, pieces of tile were introduced into the spawning tanks to facilitate spawning. Daily water exchange (20% of total volume) and tank bottom cleaning was performed. All tanks were provided with uniform aeration. The parents were retained in the breeding tank after spawning until the fourth day post-spawning (dps). Water quality parameters were monitored weekly during the breeding and larval rearing time.
The larvae rearing was done in a clear water system, since the yolk sac absorption and parental care were continued until the third dps, no external feeding was provided. From 4th dps onwards the larvae were provided with artemia nauplii at 5 numbers/ml rearing volume. From 10th dps artemia nauplii were gradually replaced with artemia flakes at 0.5 g/day/tank.
Statistical Analysis
Data presented in the text, figures, and tables are expressed as mean ± standard error. Statistical significance for various parameters was fixed at p<0.05. The mean values were compared using Student’s t-test for significant difference (p<0.05) among the stunted and normal fish using SPSS 16.0 VERSION.
Results
Evaluation of the Characteristics of the Species to be a Model Organism
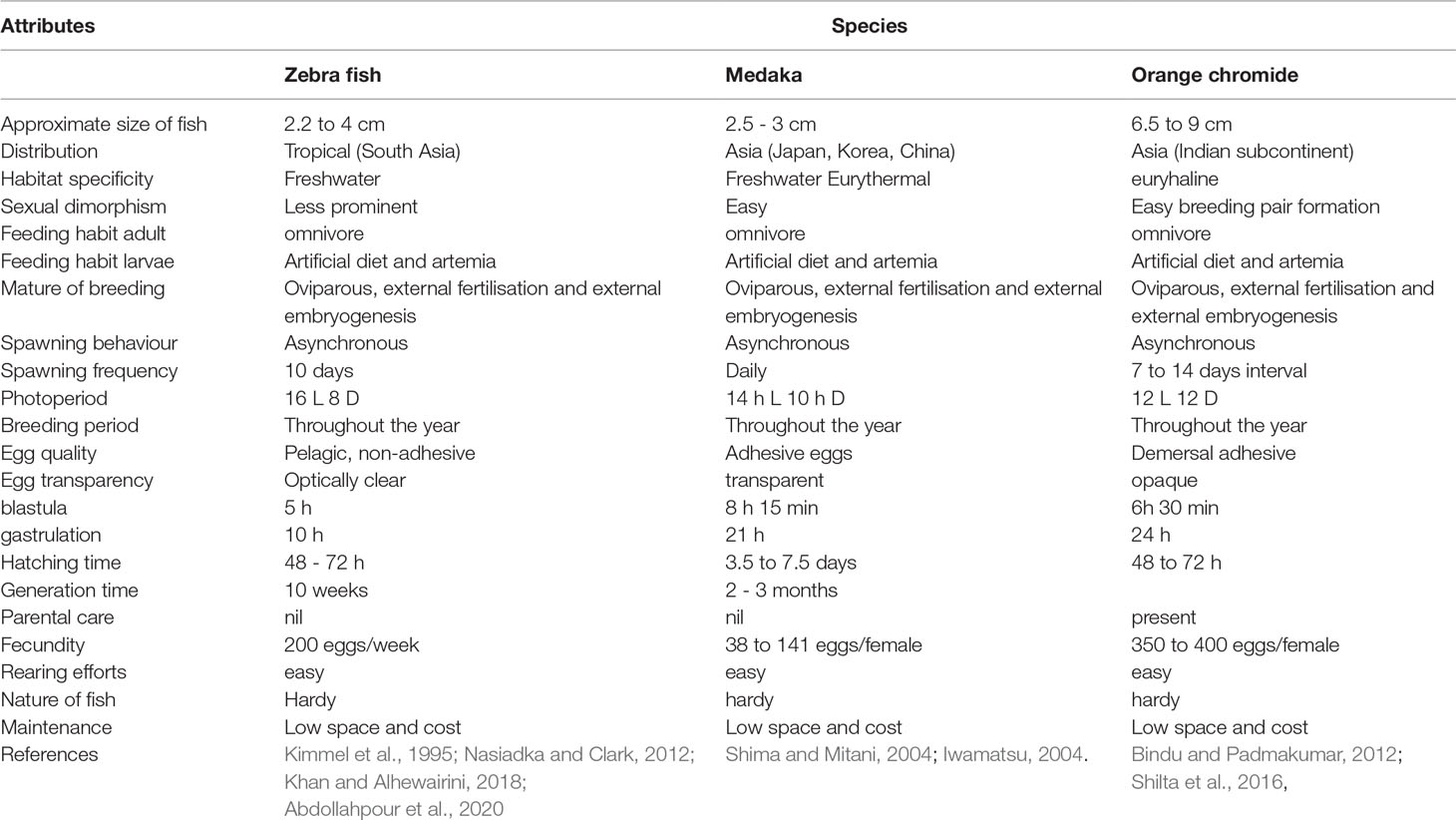
Among the important steps involved in the development of a new model organism described by Matthews and Vosshall (2020) (Table 1.) initial two steps such as choosing the organism and rearing the organism in laboratory conditions were attempted in the study. In addition, some of the important applications of the model organism (step 10) such as salinity tolerance and breeding adaptations in different salinities also were attempted. For the selection of the organism, the major characteristics of the fish were compared with established model fishes and the results are given in Table 2. Attributes pertaining to size, feeding habits, breeding behaviour, embryo development, and larval rearing almost matched well-established model fishes such as Zebrafish and Medaka.
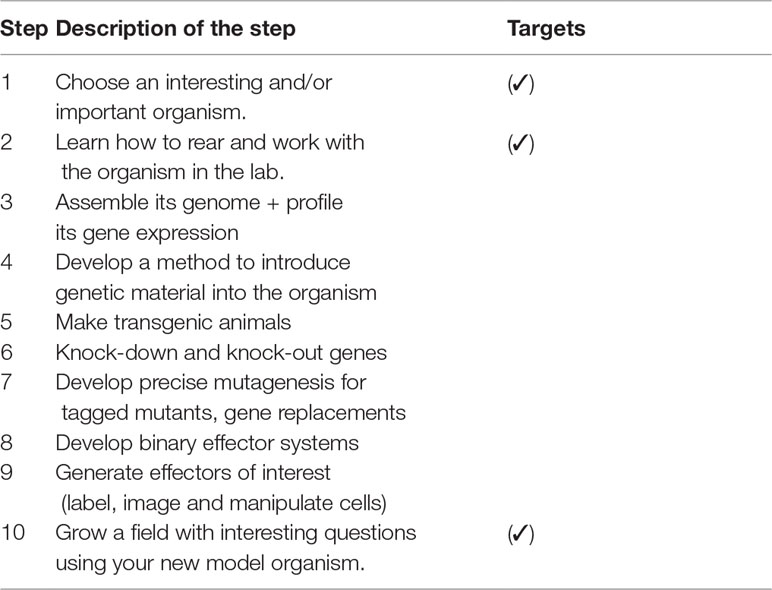
Table 1 Important steps involved in the development of a new model organism as described by Matthews and Vosshall (2020) and the targets for the present work.
Morphometric measurements and meristic counts of 50 P. maculatus samples collected from Akalapuzha, Calicut, India are given in Table 3. The prominent characters observed in the specimens were as follows: Body with 1- 4 black blotches on the dorso- posterior half of the body; anal and pelvic fins with dark black border; orange colour small dots more prominent in the mid-portion of the body and in the dorsal fins. Meristic characters: Dorsal fin spines: XVIII-XIX (mostly XVIII); Dorsal fin soft rays: 8- 10 (mostly 8-9); Anal fin spines: XII-XIV (mostly XII- XIII); Anal fin soft rays: 8-9 (mostly 8); Pectoral fin- I, 12- 13; pelvic fin- I, 5. Metric characters: Standard Length (SL) (mm)-55.51; % SL: Head Length-34.1; orbit diameter-9.7; Caudal peduncle length-8.1; Caudal peduncle width-15.0.
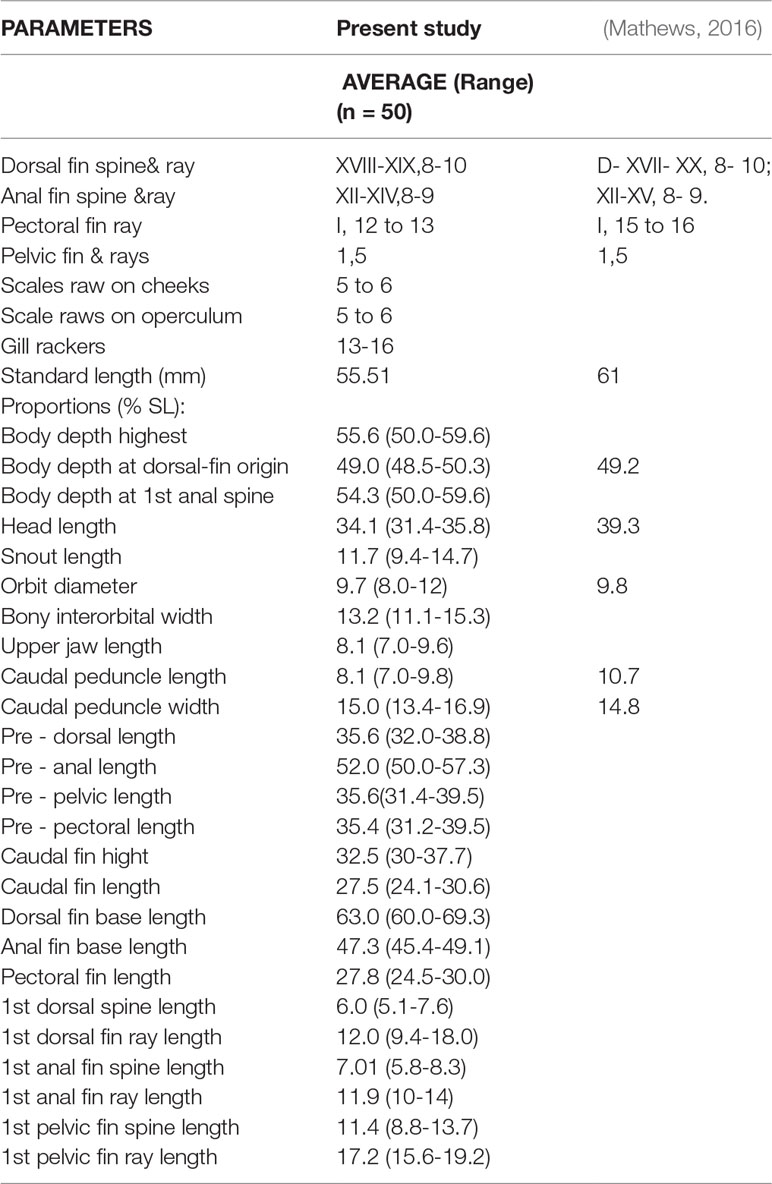
Table 3 Comparison of the counts and morphometric data of P. maculatus collected from Korapuzha river, Calicut, Kerala with P. maculatus collected from Manimala river, Travancore, Kerala (Mathews, 2016).
Key identification features of P. maculatus were derived in comparison with Etroplus suratensis and given in Figure 1. Figure 1A shows E. suratensis with a blunt snout with a steeply sloping profile; a prominent black patch on the pectoral fin and (Figure 1B) P. maculatus with a snout profile markedly acute. Figure 1C shows E. suratensis with seven prominent dark lateral bars and (Figure 1D) indicate an absence of bars but the presence of one or more black blotches on the side of the body in P. maculatus. Figure 1E shows lateral oral dentition unicuspid, spatulate in E. suratensis while Figure 1F shows a tricuspid tooth pattern in P. maculatus The sequence analysis confirmed the identity of P. maculatus which is used as a model organism in this study. The Bayesian phylogram (Figure 2) indicated two clusters in which the monotypic genus Pseudetroplus formed a sister clade to the genus Etroplus and showed 17.8% and 19.3% p-distance with E. suratensis and E. canarensis respectively. The interspecific distance in genus Etroplus was 15.7%. The comparative genetic analysis of these three South Asian cichlids revealed that they are evidently discriminated by their genetic distances and also by the criterion of cluster formation of COI barcodes in the phylogenetic tree.

Figure 1 Key identification points for P. maculatus in comparison with E suratensis (A) E suratensis with blunt snout with a steeply sloping profile; a prominent black patch on the pectoral fin (B) P. maculatus with snout profile markedly acute; pectoral fin hyaline. (C) E suratensis with seven prominent dark lateral bars (D) an absence of bars but the presence of one or more black blotches on the side of the body in P. maculatus. (E) Lateral oral dentition unicuspid, spatulate in E suratensis (F) Tricuspid in P. maculatus.
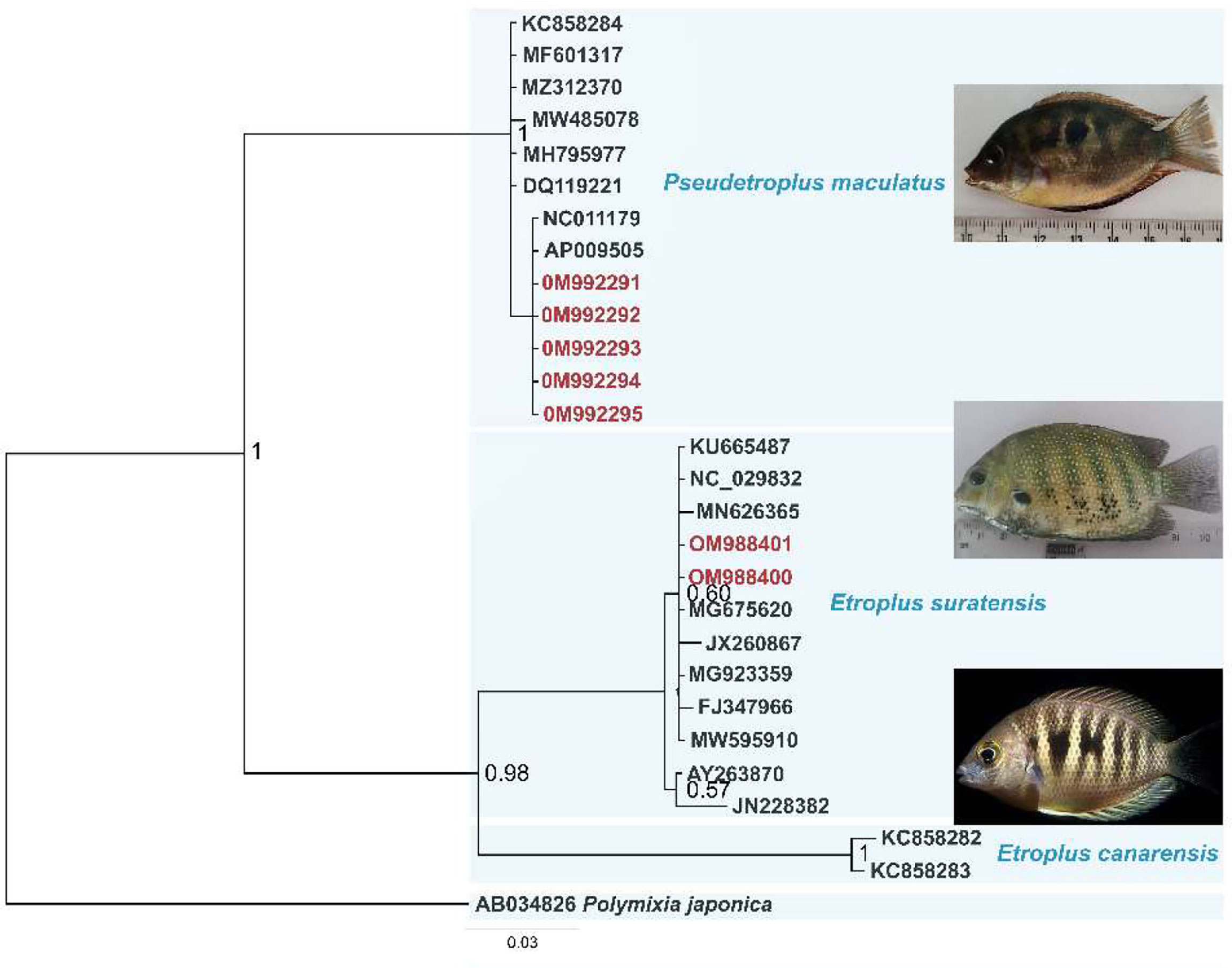
Figure 2 Bayesian phylogenetic tree based on COI sequences retrieved from GenBank (accession numbers indicated) and the specimens from this study (red text). Branch labels are posterior probability and the scale bar is the number of substitutions per site. Polymixia japonica is used as the outgroup.
Biology of the Species
The sex ratio observed for the collected stock was 1: 1.76 (male: female). Length-weight relationship of orange chromide, P. maculatus was investigated from 100 specimens collected from Akalapuzha estuary, Calicut, Kerala, India. The fish collected ranged from 4.7 - 9. 5 cm (mean 7.15 ± 0.95) in total length and 1.9 – 12.6 g (6.99 ± 2.56) in weight. Length-weight relationships with the descriptive regression parameters of the equations are presented in Table 4. Length-weight relationship for males and females and the total sample population were determined as W=0.1605L3.1172, W=0.3075L2.3418, and W=0.2396L2.6347 respectively. The overall b value ranged from 2.3 to 3.1 with a mean value of 2.63 ± 0.02. This indicated a negative allometric growth pattern (b<3) in P. maculatus and the r2 values ranged from 0.73 to 0.91. The condition factor for males was 1.98 and for females it was 1.74, overall condition factor was 1.91.
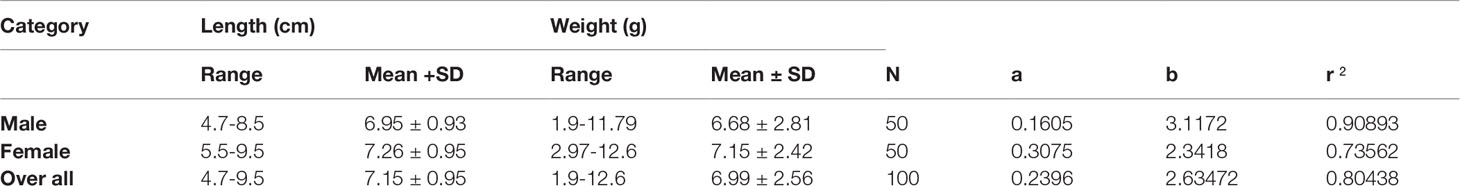
Table 4 Descriptive statistics and estimated parameters of Length-weight relationships for P. maculatus collected from the Korapuzha estuary.
Biopsy samples of the ovary of P. maculatus sample from an ovary (Figure 3) revealed the presence of developing oocytes (small spherical), immature oocytes (small oval), and ripe oocytes (large oval) in a single ovary indicating asynchronous development of oocytes in the ovary. Details of the maturation stages of oocytes observed in femaleP. maculatus are given in Table 5. As the oocyte matures the shape changes from spherical to oval with yolk deposition and the oocyte grows from an average diameter of 181.5 ± 10.64 µm to a size of 845.92 ± 57.39 x 1159.96 ± 59.6 µm.
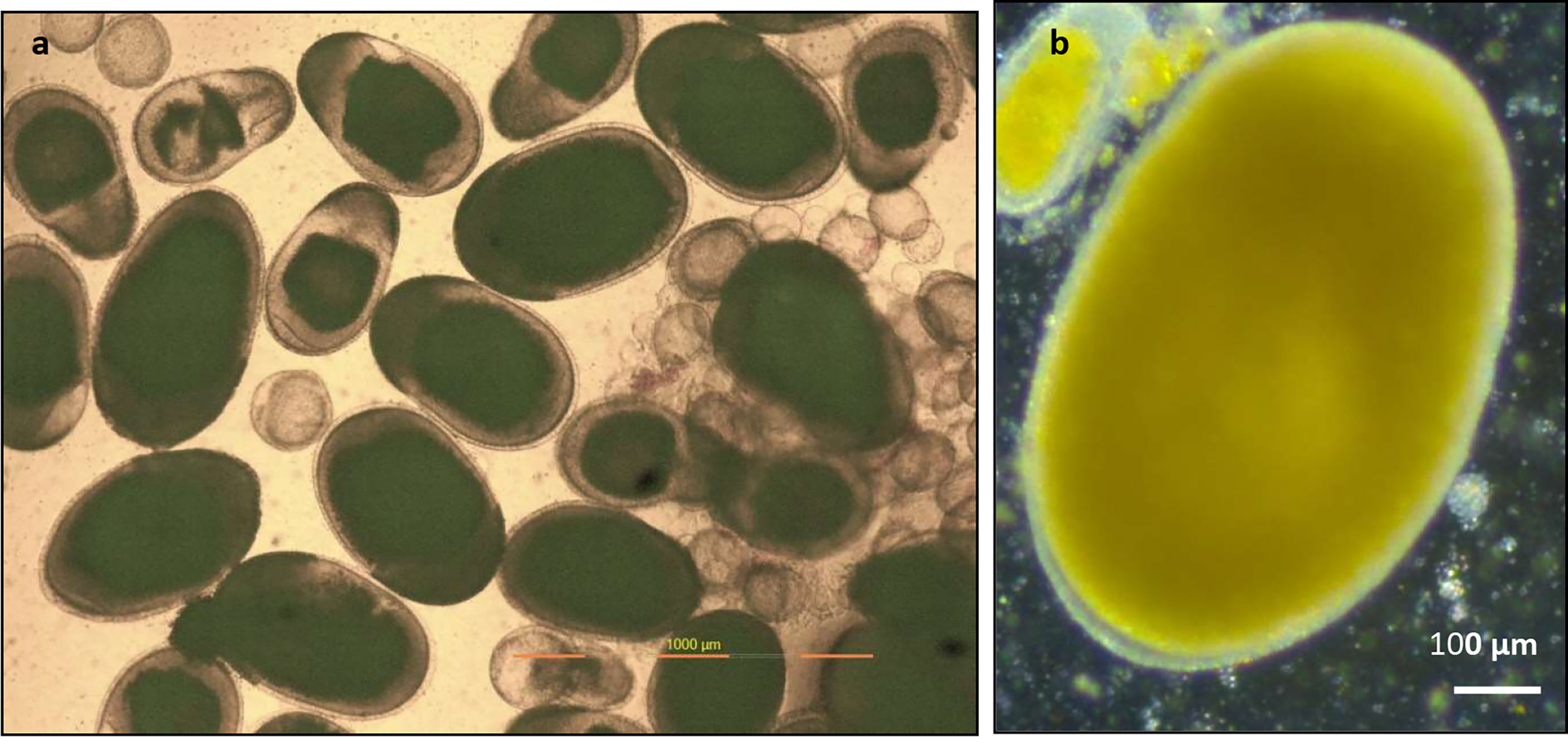
Figure 3 Biopsy samples of ovary of P. maculatus (A) sample from a female ovary indicating developing oocytes (small spherical), immature oocytes (small oval), and ripe oocytes (large oval) observed under a compound microscope. (B) image of a ripe oocyte with clear zona radiata and cytoplasm filled with yolk granules observed under a steriozoom microscope.

Table 5 Maturation stages of oocytes observed in female P. maculatus based on oocyte dimensions and colour.
Salinity Tolerance Study
The fish could be successfully reared in different salinities 0, 15, and 35 ppt and the fishes have attained a final weight of 9.4 ± 0.12 g, 8.6 ± 0.18 g, 8.2 ± 0.08 g (p>0.05) respectively from the initial average weight of 5.4 ± 0.08 g. The average survival of the fish (98%, 96%, and 96% respectively for 0%, 15%, and 35% ppt also was not different among different salinities. The fish when exposed for long periods to higher salinities at 28.5C, exhibited no change in survival and no sign of loss of appetite was observed, which is an indication that there is no imbalance in the physiological mechanisms of the animals.
Biochemical indices in the liver and serum of orange chromide reared in different salinities are given in Table 6. In liver tissue the maximum level of triglycerides was found in the 15 ppt salinity group, while the lowest was found in the 35 ppt group, while the highest level of triglycerides in serum was found in the 0 ppt group, followed by the 35 and 15 ppt groups. In the present study, the highest level of cholesterol was found in liver tissue in the 15 ppt group, while the lowest level was found in the serum in the 15 ppt group. The protein concentration of the liver was highest in the 15 ppt group, followed by the 0 and 35 ppt groups in our study. The highest amount of protein in serum was found in the 0 and 35 ppt groups, with no significant differences between them, followed by the 15 ppt group. In the current study, the 35 ppt group had the highest ALT activity in liver tissue, while the 0 and 15 ppt groups had no significant changes. In serum, the 15 ppt group had the highest ALT activity, with no significant differences between the 0 and 35 ppt groups. In liver tissue, AST activity was highest in the 15 and 35 ppt groups, whereas in serum, it was highest in the 20 ppt group, followed by 0 ppt and 35 ppt groups.
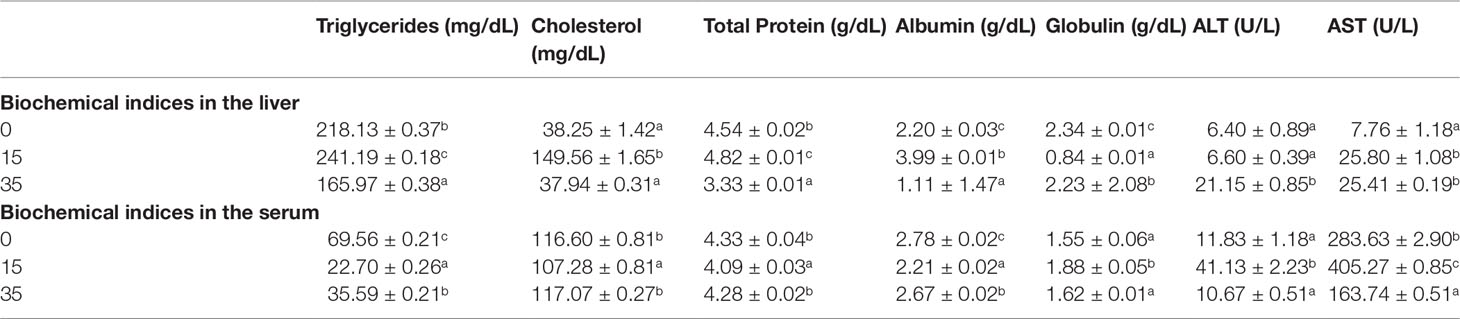
Table 6 Biochemical indices in the liver and serum of Orange chromide reared in different salinities.
Variation in GSI and HSI in P. maculatus reared in different salinities at the end of the experiment is given in Figure 4. GSI and HSI were showing similar trends in different salinities with highest values in fish reared in 15 ppt and lower values for the other salinities.
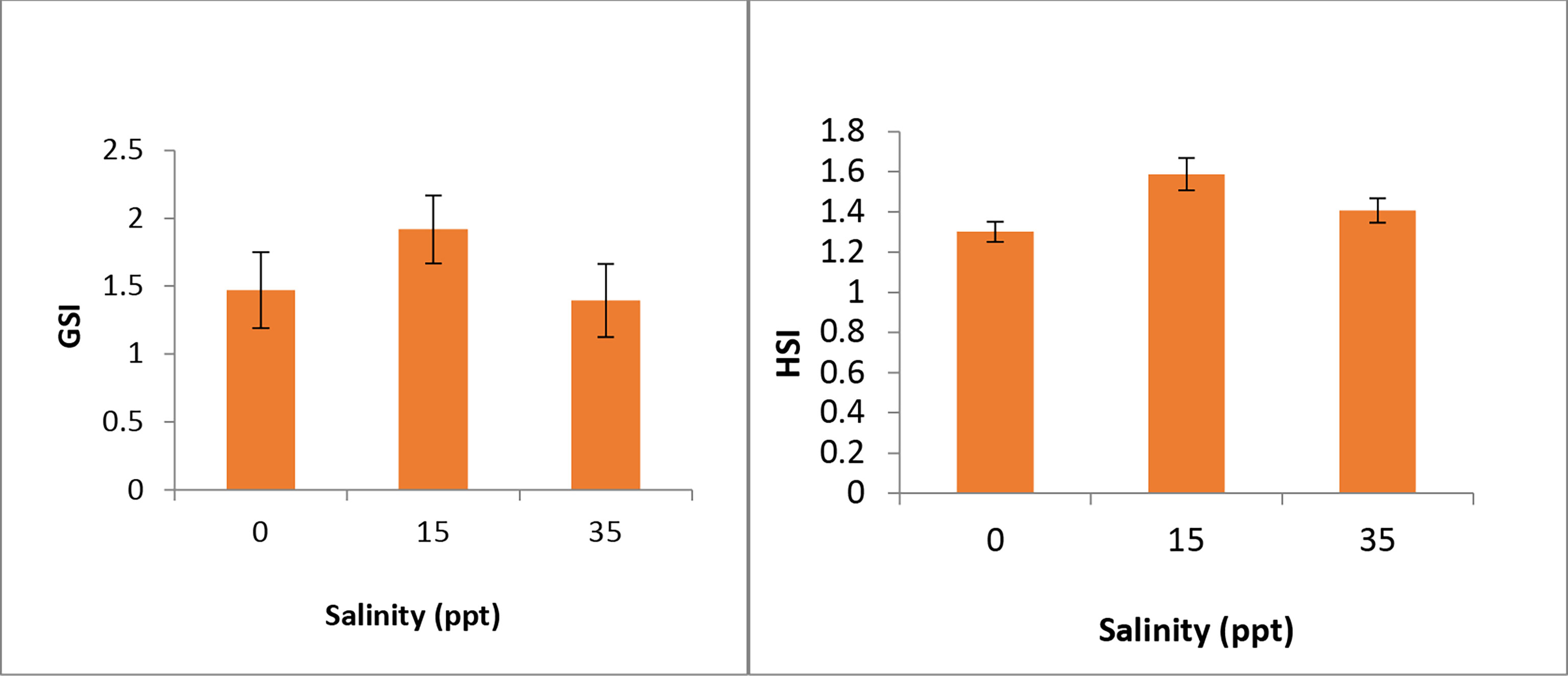
Figure 4 Variation in Gonado-somatic index (GSI) and Hepato-somatic index (HSI) in P. maculatus reared in different salinities.
Breeding in Different Salinities
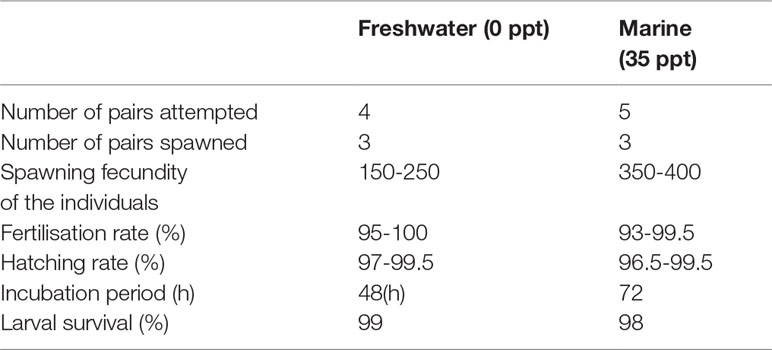
Spawning was observed in both the salinities maintained. Details of the spawning obtained are given in Table 7. The process of spawning is completed within 15- 30 minutes in all the salinities. The higher spawning fecundity about 350 to 400 eggs per female was observed in 35 ppt (Figure 5) when compared to freshwater (150-250 eggs/female). After hatching most of the hatchlings remained in the substrate itself. The hatchlings were found to sink to the bottom after a few hours and they were mouth-picked by the female and transferred to one of the darker corners of the plastic crates. The hatchlings had heads down and tails up with lashing movements. The newly hatched larvae appeared transparent with a voluminous yolk sac containing large oil globules; presence of large pigmented eyes; prominent pulsing heart located between the head and yolk sac. Yolk absorption was completed in three days, after which the larvae began to accept external food. In a few tanks, it was observed that the two day old larvae were devoured by the parents and they are promptly removed immediately from each spawning tank. The fry become free swimming from the fourth day and are found congregated near the aeration points as swarms in tanks due to the absence of parents in the tanks. In general, fry move in shoals guided by the parents, swimming mostly underneath the parents. From the fourth day immediately after the yolk sac absorption, fry was fed with artemia nauplii, since the yolk sac absorption was completed. From the tenth day, the amount of artemia nauplii is reduced and artemia flakes were added to the tanks. After 25 days of rearing in fresh water and seawater a survival rate of 98 - 99% was observed.

Figure 5 Breeding of P. maculatus in different conditions. P. macuIatus eggs attached to a substrate in (A) Freshwater and (B) Marine water. One day old hatchlings of P. maculatus in (C) Freshwater (D) Marine water.
Discussion
Fish are considered as one of the most suitable model organisms for experimental studies. Fish are denoted as an ideal model because of their minimum inputs for maintenance and their ability to produce isogenic and genetically modified lines (Cossin and Crawford, 2005). Because fish live in water, the close physiological relationship with the environment is more intact than in the terrestrial animals making them more appropriate for environmental interaction studies (Cossin and Crawford, 2005). This advantage makes fish an ideal organism to study the impact of climate change on biological processes.
Size is an important factor in the selection of a model organism (Ulloa et al., 2011). Since P. maculatus grows only up to 7 to 9 cm (Bindu and Padmakumar, 2012; Shilta et al., 2016), it can be easily maintained in laboratory facilities similar to other model fishes. Smaller fishes such as Zebrafish, Danio rerio (Dai et al., 2014), Medaka, Oryzias latipes (Wittbrodt et al., 2002), and Goldfish, Carassius auratus (Popesku et al., 2008) have been regularly employed as model organisms for several studies. The omnivorous nature of feeding habits of both adults and larvae, and acceptance of artificial feed and live feed by the larvae facilitate rearing in confined conditions. Higher fecundity and hatching time (Shilta et al., 2016) and asynchronous spawning with a shorter spawning frequency of one or two weeks (Bindu and Padmakumar, 2012) makes the fish more ideal to be an experimental animal. Easy pair formation and parental care are unique for this fish. Since the eggs are adhesive in nature handling for the purpose of shifting and incubation is easier. Practical aspects of natural breeding such as lower space requirement, adaptability to environmental conditions, and easy feed management (Matthews and Vosshall, 2020) make P. maculatusan ideal candidate to become a model fish. Since all these characteristics are consistent with the established model fishes such as Zebrafish (Kimmel et al., 1995; Nasiadka and Clark, 2012; Khan and Alhewairini, 2018; Abdollahpour et al., 2020) and Medaka (Shima and Mitani, 2004; Iwamatsu, 2004), P. maculatus can be considered as a potential candidate to become a model fish.
Species Identification
Orange chromide was placed in the genus Etroplus until Pethiyagoda et al. (2014) validated the new genus Pseudetroplus. Presently the species is renamed Pseudetroplus maculatus. In order to confirm the identity of the specimens collected for the experiments, conventional taxonomic identification using morphometric and meristic characters and identification employing DNA Barcoding was adopted.
Sparks (2008) reported that adults of E. suratensis and E. canarensis differ from P. maculatus by possessing a blunt snout with a steeply sloping profile against the snout profile markedly acute in P. maculatus; a prominent black patch on the pectoral fin, near its base in E. suratensisagainst pectoral fin hyaline in P. maculatus and seven to nine prominent dark lateral bars against an absence of bars but the presence of one or more black blotches on the side of the body in P. maculatus. All these characters mentioned for P. maculatus were observed in the specimens collected from Akalapuzha. Bleeker (1862) recognized a higher-level distinction between P. maculatus and E. suratensis, on the basis that P. maculatus possesses tricuspid jaw teeth and scaly sheaths to the dorsal and anal fins. On contrary, Günther (1862), was doubtful of the validity of the genus Pseudetroplus, stating that the characters of the new genus Pseudetroplus mentioned by Bleeker (1862) are equally developed in E. suratensis and in E. maculatus, and concluded that Bleeker (1862) either described a third species, different from both, or that the author has taken the characters for Etroplus from a very old specimen of E. suratensis, in which the incisions on the front teeth have become obsolete. Our results on the dentition pattern were consistent with the description of Bleeker (1862) especially the presence of tricuspid teeth in jaws in P. maculatus. Similarly, Pethiyagoda et al. (2014) also reported the anterior jaw teeth tricuspid, acuminate in Pseudetroplus against the unicuspid, and spatulate teeth in Etroplus.
The morphometric measurements and meristic counts of the specimens collected in the present study were similar to those specimens reported from Manimala river, Travancore, Kerala, India with few variations (Mathews, 2016). The variations observed in comparison with specimens from the Manimala river are the counts of dorsal-fin spines (18-19 vz 17-20); anal-fin spines (12– 14 vz 12-15); pectoral-fin rays (12- 13 vz 15- 16) (Mathews, 2016). Day (1877) reported that specimens of P. maculatus from Madras, Tamil Nadu, India possessed 17–18 dorsal-fin and 11–12 anal-fin spines, whereas those in southern Karnataka, India possessed 19–20 and 14–15 spines, respectively. In the present study P. maculatus possessed 18-19 dorsal-fin spines and 12-14 anal fin spines. The difference observed in morphometric and meristic characters owes to the origin of species from two different geographical regions or genetic differences or environmental impact, or a combination of all the above.
Confirmation of the species identification was carried out using molecular tools. The sequence analysis confirmed the identity of P. maculatus. The Bayesian phylogram indicated two clusters in which the monotypic genus Pseudetroplus formed a sister clade to genus Etroplus and showed 17.8% and 19.3% p-distance with E. suratensis and E. canarensis respectively. The interspecific distance in genus Etroplus was 15.7%. The comparative genetic analysis of these three South Asian cichlids revealed that they are evidently discriminated by their genetic distances and also by the criterion of cluster formation of COI barcodes in the phylogenetic tree. Finally, the identity of the specimens collected was confirmed as P. maculatus.
Biology of the Species
Sexual dimorphism is not conspicuous in the species during the breeding season but the natural pair formation will help to identify breeding pairs for experimental breeding programmes. Bindu and Padmakumar (2012) reported intensified colouration in males during the pair formation. The fish is monogamous (Bindu and Padmakumar, 2012) and therefore it is easier to maintain the same pairs for multiple breeding cycles. The sex ratio observed in the collected fish is female dominant (male: female 1: 1.76). Roshni and Renjithkumar (2021) reported a female dominant population from the Cochin estuary and opined that the sex ratio varies with stock.
The b values of the length-weight relationships of P. maculatus exhibited notable variations from the isometric value (Froese, 2006) and all values of parameter b for the P. maculatus collected from Akalapuzha varied within the range of 2.3–3.1. The values of parameter b in the LWR equation were estimated as 2.63 for P. maculatus in the present study. Similarly, Roshni et al. (2016) reported b value ranging from 2.662 to 2.794 for P. maculatus from the Vembanad lake, Kerala, India and Remya et al. (2021) reported b value ranging from 2.779 to 3.012 for P. maculatus from the Kayamkulam lake in Kerala, India. Karna et al. (2012) also reported similar results for fish collected from Chilka lake, India.
The average relative length of gut in P. maculatus is measured to be 2.08 ± 0.27 cm. Taki (1978) suggested that fish having a relative length of digestive tract shorter than 1.5 times the standard length were judged to be carnivorous and those with a relative length of digestive tract longer than three times the standard length were regarded as herbivorous. This ratio is intermediate between 2 and 3 times the standard length. Therefore, the feeding habit of P. maculatus could be considered as an omnivore. Condition factor (above 1) for male, female, and overall groups indicates that the fish have grown in good condition. Condition factor ‘K’ is a good indicator of the degree of the well-being of the fish in their habitat based on the isometric growth pattern (Gomiero and Braga, 2005; Datta et al., 2013).
Biopsy samples of ovary of P. maculatus (a) sample from a female ovary revealed the presence of developing oocytes (small spherical), immature oocytes (small oval), and ripe oocytes (large oval) in a single ovary indicating asynchronous development of oocytes in the ovary. Thus, P. maculatus is a multiple spawner with a shorter spawning interval. Bindu and Padmakumar (2012) reported a spawning interval ranging from 8 to 16 days in different pairs of the species in freshwater. Roshni and Renjithkumar (2021) reported the presence of ripe gonads throughout the year indicating the possible spawning throughout the year. The histological section of ovary of P. maculatus depicted by Sajla et al. (2019) also represented oocytes of various developing stages on a single section of the ovary. Lamon and Ward (1983) also reported repeated spawning in P. maculatus. As the oocyte matures, the shape changes from spherical to oval with yolk deposition and the oocyte grows from an average diameter of 181.5 ± 10.64 µm to a size of 845.92 ± 57.39 x 1159.96 ± 59.6 µm. Selman et al. (1993) reported a diameter of about 0.75 mm for the matured oocytes of Zebrafish. Iwamatsu (2004) reported an oocyte diameter of above 800 µm for medaka. The bigger size of the matured oocytes poises greater advantages for the model organism since micromanipulation of the oocytes can be managed with greater precision.
Absolute fecundity obtained in the present study was 350 to 500 eggs/female. Roshni and Renjithkumar (2021) reported an absolute fecundity of 508 -830 for P. maculatus. The absolute fecundity was ideal for the continuous breeding of the species.
Salinity Tolerance Studies
In order to confirm the salinity tolerance of the species, the fish were directly exposed to various salinities in freshwater (0 ppt), brackishwater (15 ppt), and marine conditions (35 ppt). The growth and survival of the fish in different salinities did not differ much indicating the ability of the fish to perform well in all these salinities. Pampapathi Rao (1958) and Virabhadrachari (1961) reported the ability of this species to thrive in extreme salinities ranging from freshwater to salinity up to 100 ppt. According to Parvatheswararao (1967) this euryhaline nature may perhaps be due to the high blood chloride content and the high blood-tissue chloride gradient in blood-tissue.
Haemato-biochemical parameters are indications of fish health in general (Soltanian and Fereidouni, 2019). In this study, in liver tissue the maximum level of triglycerides was found in the 15 ppt salinity group, while the lowest was found in the 35 ppt group, while the highest level of triglycerides was found in the 0 ppt group, followed by the 35 and 15 ppt groups in serum. Triglycerides increased in shi drum between 4 and 10 ppt (3.1 to 7.1 mM, respectively) according to Mylonas et al. (2009), but dropped at 40 ppt (4.7 mM). Arjona et al. (2009), on the other hand, observed that plasma triglycerides increased in the sole, Solea senegalensis. Between 15 and 39 ppt, ranging from 2.8 to 10.7 mM.
Cholesterol is required for the growth and development of eukaryotic cells (Kim et al., 2019). Environmental stress such as temperature stress (Malekar et al., 2018) low dissolved oxygen (Maita et al., 1998), and inadequate nutrition, have all been shown to impact plasma cholesterol levels in fish (Krogdahl et al., 1999). In our study, the highest level of cholesterol was found in the liver tissue in the 15 ppt group, while the lowest levels were found in the 0 and 35 ppt groups without significant differences among them. The serum samples recorded an inverse trend with the lowest level in the 15 ppt group and higher levels in both 0 and 35 ppt groups without significant differences among them.
Total plasma protein is a reliable physiological indication of fish health status and environmental stress because it plays a role in osmotic regulation and pathogenic defence (Kim and Kang, 2016). In fish, total protein is significantly linked to a greater innate immune response (Sangiao-Alvarellos et al., 2005). Increased salinity resulted in a considerable drop in protein levels (Martinez-Alvarez et al., 2002). Riche (2007), on the other hand, found that changes in salinity enhanced the total protein of hybrid striped bass (Morone chrysops x M. saxatilis). The protein concentration of the liver was highest in the 15 ppt group, followed by the 0 and 35 ppt groups in our study. The highest amounts in the serum were found in the 0 and 35 ppt groups, with no significant differences between them, followed by the 15 ppt group.
Plasma ALT and AST are sensitive indicators of liver injury and health status in fish (Kim and Kang, 2014; Kim and Kang, 2015). In the current study, the 35 ppt group had the highest ALT activity in liver tissue, while the 0 and 15 ppt groups had no significant changes. In serum, the 15 ppt group had the highest activity, with no significant differences between the 0 and 35 ppt groups. In liver tissue, AST activity was highest in the 15 and 35 ppt groups, whereas in serum, it was highest in the 15 ppt group, followed by 0 ppt and 35 ppt groups. Under salinity stress, the AST and ALT of goldfish (Carassius auratus) was increased significantly, according to Al-Khashali and Al-Shawi (2013). In low-salinity environments, Lee et al. (2016) found a substantial rise in plasma AST of juvenile red-spotted grouper (Epinephelus akaara), implying that hepatic or cardiomyocyte function may be compromised. The GSI and HSI values in different salinities after 45 days of rearing were slightly higher in the 15 ppt group indicating higher reproductive activity in this salinity. The higher biochemical indices in the liver in the 15 ppt group also indicate the same.
Breeding in Different Salinities
Spawning was observed in both the salinities maintained. The process of spawning is completed within 15- 30 minutes in both the salinities. The higher spawning fecundity of approximately 350 to 400 eggs per female was observed at 35 ppt when compared to freshwater (150-250 eggs/female). Bindu and Padmakumar (2012) reported that spawning fecundity varied between 140 to 231 per female in freshwater. The mean fecundity in P. maculatus was reported to be 1,378 (Jayaprakas et al., 1979). However, Antony and Natarajan (2014) reported a fecundity ranging from 250 to 400 for the species in freshwater indicating less correlation between the fecundity and salinity of the water in which breeding trials were performed. The difference in spawning fecundity might be due to the ‘asynchronous’ development of oocytes in the ovary of P. maculatus and multiple batches of eggs were spawned successively within a spawning season. After spawning, both parents alternately guard over eggs by fanning and mouthing, while the other leaves the territory to forage. Their roles were reversed in every few minutes and this keep the pairs in good health (Perrone and Zaret, 1979). During the experiment, in a few tanks where the brooders were disturbed, they themselves consumed the developing eggs. Bindu and Padmakumar (2012) also reported similar behaviour. The eggs of P. maculatus in fresh water hatched out in 48 hours whereas in saline water eggs hatched within 68 to 72 hours. Similarly, Bindu and Padmakumar (2012) reported that the eggs of P. maculatus generally hatched out in 48 hours in freshwater. Parental care ensured high hatching up to 97-99.5% in freshwater and 96.5-99.5% in marine water. Biparental care is reported for this species by several authors (Bindu and Padmakumar, 2012; Shilta et al., 2016). Bindu and Padmakumar (2012) reported a 99% hatching in freshwater with parental care and reported a reduced hatching rate without parental care. Yolk absorption was completed in three days and after which the larvae began to accept external food. The experiment revealed that the species can be bred in both freshwater and marine conditions.
P. maculatus as a Model Fish to Study Climate Change
P. maculatus is considered as an euryhaline species (Virabhadrachari, 1961) which inhabits mostly freshwater and backwaters in the Indian subcontinent. This species has been considered as an excellent ornamental and food fish in this region (Bindu and Padmakumar, 2012; Raghavan et al., 2013; Shilta et al., 2016) since breeding in nature and in captivity is easy. In addition, the present work revealed that the species can tolerate, mature, and reproduce in salinity regimes ranging from freshwater to marine conditions with minimum physiological alterations. These characteristics along with the specific characteristics similar to that of a model organism, such as ease of breeding, easy stock management, and prolonged breeding season make the species an ideal candidate to study the influence of fluctuating climate parameters such as altered temperature, pH, dissolved oxygen level, osmoregulation and nutrient enhancement in different salinity regimes. Changes in metabolic and other physiological functions, reproductive behavior, stress parameters, and disease manifestation in different salinity regimes according to climate changes can be addressed with the new model organism. This will help fish farmers to adopt strategies to mitigate the impact of climate change on fish growth, production, and reproduction.
Conclusion
The present work indicates that the euryhaline fish P. maculatus is an ideal candidate to become a model organism for studying the changes in habitats, as it thrives and reproduces well in all the salinity regimes. It’s worth noting that, while salinity had an effect on the biochemical indices of the liver and serum, the ultimate growth, breeding performance, and survival were unaffected. This demonstrates the species’ ability to adapt to a wide range of salinity. Moreover, the fish fulfil most of the criteria to be a model organism similar to the established fish models employed for several biological studies.
Data Availability Statement
The data presented in the study are deposited in the NCBI GenBank repository(https://www.ncbi.nlm.nih.gov/), accession numbers OM992292-OM992296 and OM988400-OM988401.
Ethics Statement
This animal study was reviewed and approved by ICAR-CMFRI, Kochi, India (Project code MDN/HCY/18).
Author Contributions
SP, Concept, Manuscript preparation, data analysis. ST, execution of work, manuscript preparation. AA, Data analysis. JS, execution of work, manuscript preparation. AK, execution of work. SE, execution of work, Data analysis. BI, Manuscript preparation. GA. Over all guidance. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors are grateful to the Director, Central Marine Fisheries Research Institute, Kochi, for the support and encouragement provided for carrying out the research work.
References
Abdollahpour H., Falahatkar B., Lawrence C. (2020). The Effect of Photoperiod on Growth and Spawning Performance of Zebrafish, Danio Rerio. Aquac. Rep. 17, 100295. doi: 10.1016/j.aqrep.2020.100295
Al-Khashali M. S., Al-Shawi S. A. S. (2013). Effect of Salt Stress on ALT and AST Enzymes Activity and Cortisol Level in Adults of Carassius Auratus. Pak. J. Nutr. 12, 97–100. doi: 10.3923/pjn.2013.97.100
Ankeny R. A., Leonelli S. (2020). “Model Organisms Elements,” in The Philosophy of Biology. United Kingdom: Cambridge University Press,doi: 10.1017/9781108593014
Antony J., Natarajan P. (2014). An Analysis of the Significance of Certain Factors That Affect the Breeding and Survival of Etroplus Maculatus. J. Aquat. Biol. Fish. 2 (1), 150–153.
Arjona F. J., Vargas-Chacoff L., Ruiz-Jarabo I., Gonçalves O., Pascoa I., Martin Del Rio M. P., et al. (2009). Tertiary Stress Responses in Senegalese Sole (Solea Senegalensis Kaup 1858) to Osmotic Challenge: Implications for Osmoregulation, Energy Metabolism and Growth. J. Aquacult. 287, 419–426. doi: 10.1016/j.aquaculture.2008.10.047
Bindu L., Padmakumar K. G. (2012). Breeding Behaviour and Embryonic Development in the Orange Chromide, Etroplus Maculatus (Cichlidae, Bloch 1795). J. Mar. Biol. Ass. India. 54 (1), 13–19. doi: 10.6024/jmbai.2012.54.1.01679-02
Bleeker P. (1862). Notices Ichthyologiques (I–X). Verslagen En Mededeelingen Der Koninklijke Akademie Van Wetenschappen. Afdeling Natuurkunde. 14, 123–141.
Cossin A. R., Crawford D. L. (2005). Fish as Models for Environmental Genomics. Nat. Rev. Genet. 6 (4), 324–333. doi: 10.1038/nrg1590
Dai Y. J., Jia Y. F., Chen N., Bian W. P., Li Q. K., Ma Y. B., et al. (2014). Zebrafish as a Model System to Study Toxicology. Environ. Toxicol. Chem. 33 (1), 11–17. doi: 10.1002/etc.2406
Datta S. N., Kaur V. I., Dhawan A., Jassal G. (2013). Data From: Estimation of Length-Weight Relationship and Condition Factor of Spotted Snakehead Channa Punctata (Bloch) Under Different Feeding Regimes. Springerplus. doi: 10.1186/2193-1801-2,436
Day F. (1877). The Fishes of India: Being a Natural History of the Fishes Known to Inhabit the Seas and Fresh Waters of India, Burma, and Ceylon, Part 3 (London: William Dawson & Sons).
Ebeneezar S., Vijayagopal P., Srivastava P. P., Gupta S., Varghese T., Linga Prabu D., et al. (2020). Optimum Dietary Methionine Requirement of Juvenile Silver Pompano, Trachinotus Blochii (Lacepede 1801). Anim. Feed Sci. Technol. 268, 114592. doi: 10.1016/j.anifeedsci.2020.114592
Euzen O. (1987). Food Habits and Diet Composition of Some Fish of Kuwait. Kuwait. Bull. Mar. Sci. 9, 65–85.
Froese R. (2006). Cube Law, Condition Factor and Weight–Length Relationships: History, Meta-Analysis and Recommendations. J. Appl. Ichthyol. 22, 241–253. doi: 10.1111/j.1439-0426.2006.00805.x
Gomiero L. M., Braga F. M. S. (2005). The Condition Factor of Fishes From Two River Basins in Sao Paulo State, Southeast of Brazil. Acta Sci. 27, 73–78. doi: 10.4025/actascibiolsci.v27i1.1368
Günther A. (1862). Catalogue of the Fishes in the British Museum: Catalogue of the Acanthopterygii, Pharyngognathi and Anacanthini in the Collection of the British Museum Vol. 4 (London: British Museum).
Huelsenbeck J. P., Ronquist F. (2001). MRBAYES: Bayesian Inference of Phylogenetic Trees. Bioinformatics. 17 (8), 754–755. doi: 10.1093/bioinformatics/17.8.754
Iwamatsu T. (2004). Stages of Normal Development in the Medaka Oryzias Latipes. Mech. Dev. 121 (7-8), 605–618. doi: 10.1016/j.mod.2004.03.012
Jayaprakas V., Padmanabhan K. G., Balasubramanian N. K. (1979). “Food, Feeding Habits and Breeding Biology of the Orange Chromide Etroplus Maculatus (Bloch),” in Bulletin of the Department of Aquatic Biology, vol. 4. (India: University of Kerala), 9–21.
Jayaram K. C. (2010). Fresh Water Fishes of the Indian Region (New Delhi: Narendra publishing House).
Karna S. K., Sahoo D., Panda S. (2012). Length Weight Relationship (LWR), Growth Estimation and Length at Maturity of Etroplus Suratensis in Chilika Lagoon, Orissa, India. Int. J. Environ. Sci. 2 (3), 1257–1267. doi: 10.6088/IJES.00202030012
Khan F. R., Alhewairini S. S. (2018). “Zebrafish (Danio Rerio) as a Model Organism” in Current Trends in Cancer Management. Eds. Streba L., Gheonea D. I., Schenker M. (London: Intech Open). doi: 10.5772/intechopen.81517
Kim J. H., Kang J. C. (2014). The Selenium Accumulation and its Effect on Growth, and Haematological Parameters in Red Sea Bream, Pagrus Major, Exposed to Waterborne Selenium. Ecotoxicol. Environ. Saf. 104, 96–102. doi: 10.1016/j.ecoenv.2014.02.010
Kim J. H., Kang J. C. (2015). The Lead Accumulation and Hematological Findings in Juvenile Rock Fish Sebastes Schlegelii Exposed to the Dietary Lead (II) Concentrations. Ecotoxicol. Environ. Saf. 115, 33–39. doi: 10.1016/j.ecoenv.2015.02.009
Kim J. H., Kang J. C. (2016). The Chromium Accumulation and its Physiological Effects in Juvenile Rockfish, Sebastes Schlegelii, Exposed to Different Levels of Dietary Chromium (Cr6+) Concentrations. Environ. Toxicol. Pharmacol. 41, 152–158. doi: 10.1016/j.etap.2015.12.001
Kim J. H., Kim S. K., Hur Y. B. (2019). Hematological Parameters and Antioxidant Responses in Olive Flounder Paralichthys Olivaceus in Biofloc Depend on Water Temperature. J. Therm. Biol. 82, 206–212. doi: 10.1016/j.jtherbio.2019.04.013
Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B., Schilling T. F. (1995). Stages of Embryonic Development of the Zebrafish. Dev. Dyn. 203 (3), 253–310. doi: 10.1002/aja.1002030302
Krogdahl A., Nordrum S., Sorensen M., Brudeseth L., Rosjo C. (1999). Effects of Diet Composition on Apparent Nutrient Absorption Along the Intestinal Tract and of Subsequent Fasting on Mucosal Disaccharidase Activities and Plasma Nutrient Concentration in Atlantic Salmon Salmo Salar L. Aquac. Nutr. 5, 121–133. doi: 10.1046/j.1365-2095.1999.00095.x
Kumar S., Stecher G., Li M., Knyaz C., Tamura K. (2018). MEGA X: Molecular Evolutionary Genetics Analysis Across Computing Platforms. Mol. Biol. Evol. 35 (6), 1547. doi: 10.1093/molbev/msy096
Lamon M. S., Ward J. A. (1983). “Measurements of Reproductive Effort From Successive Reproductive Cycles for the Asian Cichlid Etroplus Maculates,” in Predators and Prey in Fishes (Dordrecht: Springer), 149–158.
Lee J. W., Kim H. B., Baek H. J. (2016). Plasma Stress Responses in Juvenile Red-Spotted Grouper (Epinephelus Akaara) Exposed to Abrupt Salinity Decrease. Dev. Reprod. 20, 187–196. doi: 10.12717/DR.2016.20.3.187
Maita M., Satoh K. I., Fukuda N., Okamoto N., Ikeda Y. (1998). The Influence of the Dissolved Oxygen on Levels of Plasma Components in Yellowtail. Nippon. Suisan. Gakkai. Shi. 64, 288–289. doi: 10.2331/suisan.64.288
Malekar V. C., Morton J. D., Hider R. N., Cruickshank R. H., Hodge S., Metcalf V. J. (2018). Effect of Elevated Temperature on Membrane Lipid Saturation in Antarctic Notothenioid Fish. PeerJ, 6: e4765. doi: 10.7717/peerj.4765
Martinez-Alvarez R., Hidalgo M., Domezain A., Morales A., García-Gallego M., Sanz A. (2002). Physiological Changes of Sturgeon Acipenser Naccarii Caused by Increasing Environmental Salinity. J. Exp. Biol. 205, 3699–3706. doi: 10.1242/jeb.205.23.3699
Mathews P. (2016). Systematic Accounts on Percoid Fishes of Manimala River of Travancore. Int. J. Environ. Sci. 2 (5), 12–23. doi: 10.20431/2454-9444.0205002
Matthews B. J., Vosshall L. B. (2020). How to Turn an Organism Into a Model Organism in 10 ‘Easy’ Steps. J. Exp. Biol. 223 (Suppl_1), 218198. doi: 10.1242/jeb.218198
Mylonas C. C., Pavlidis M., Papandroulakis N., Zaiss M. M., Tsafarakis D., Papadakis I. E., et al. (2009). Growth Performance and Osmoregulation in Shi Drum (Umbrina Cirrosa) Adapted to Different Environmental Salinities. Aquaculture. 287, 203–210. doi: 10.1016/j.aquaculture.2008.10.024
Nasiadka A., Clark M. D. (2012). Zebrafish Breeding in the Laboratory Environment. ILAR. J. 53 (2), 161–168. doi: 10.1093/ilar.53.2.161
National Institutes of Health (NIH) (1999) Model Organisms for Biomedical Research. Available at: www.nih.gov/science/models/.
Parvatheswararao V. (1967). Effects of Prolonged Starvation on the Metabolism of Freshwater Fishes, Etroplus Maculatus and Cirrhina Reba. Life Sci. 6), 163–172. doi: 10.1016/0024-3205(67)90301-3
Perrone P., Zaret T. M. (1979). Parental Care Patterns of Fishes. Am. Nat. 113, 351–361. doi: 10.1086/283394
Pethiyagoda R., Maduwage K., Manamendra-Arachchi K. (2014). Validation of the South Asian Cichlid Genus Pseudetroplus Bleeker (Pisces: Cichlidae). Zootaxa. 3838 (5), 595–600. doi: 10.11646/zootaxa.3838.5.9
Popesku J. T., Martyniuk C. J., Mennigen J., Xiong H., Zhang D., Xia X., et al. (2008). The Goldfish (Carassius Auratus) as a Model for Neuroendocrine Signaling. Mol. Cell. Endocrinol. 293 (1-2), 43–56. doi: 10.1016/j.mce.2008.06.017
Raghavan R., Dahanukar N., Tlusty M., Rhyne A. L., Kumar K. K., Molur S., et al. (2013). Uncovering an Obscure Trade: Threatened Freshwater Fishes and the Aquarium Pet Markets. Biol. Conserv. 164, 158–169. doi: 10.1016/j.biocon.2013.04.019
Remya R., Amina S., Bindu L. (2021). Length–Weight Relationships of Eight Fish Species From the Kayamkulam Estuary in Kerala, India. J. Aquat. Biol. Fish. 9, 29–32.
Riche M. (2007). Analysis of Refractometry for Determining Total Plasma Protein in Hybrid Striped Bass (Morone Chrysops× M. Saxatilis) at Various Salinities. Aquaculture. 264, 279–284. doi: 10.1016/j.aquaculture.2006.12.018
Roshni K., Renjithkumar C. R. (2021). Demographics and Reproductive Characteristics of Orange Chromidae, Pseudetroplus Maculatus (Bloch 1975) From Cochin Estuary, Southern India. Thalassas. 37 (2), 905–915. doi: 10.1007/s41208-021-00322-3
Roshni K., Renjithkumar C. R., Kurup B. M. (2016). Length-Weight Relationship of Two Cichlid Fish Species, Etroplus Suratensis (Bloch 1790) and Etroplus Maculatus (Bloch,1795) From Lake Vembanad, Kerala, India. J. Appl. Ichthyol 32, 1308–1309. doi: 10.1111/jai.13141
Sajla K., Raibeemol K. P., Chitra K. C. (2019). Induction of Ovarian Toxicity in the Freshwater Fish, Pseudetroplus Maculatus (Bloch 1795) After Sublethal Exposure of Dibutyl Phthalate. Int. J. Sci. Res. Biol. Sci. 6, 5. doi: 10.26438/ijsrbs/v6i5.2638
Sangiao-Alvarellos S., Arjona F. J., del Río M. P. M., Míguez J. M., Mancera J. M., Soengas J. L. (2005). Time Course of Osmoregulatory and Metabolic Changes During Osmotic Acclimation in Sparus Auratus. J. Exp. Biol. 208, 4291–4304. doi: 10.1242/jeb.01900
Selman K., Wallace R. A., Sarka A., Qi X. (1993). Stages of Oocyte Development in the Zebrafish, Brachydanio Rerio. J. morphol 218 (2), 203–224. doi: 10.1002/jmor.1052180209
Shilta M. T., Suresh Babu P. P., Vinod K. (2016). Orange Chromide (Etroplus Maculatus): A Promising Indigenous Fish for Marine Aquariums. MFIS 227, 7–9.
Shima A., Mitani H. (2004). Medaka as a Research Organism: Past, Present and Future. Mech. Dev. 121 (7–8), 599–604. doi: 10.1016/j.mod.2004.03.011
Soltanian S., Fereidouni M. S. (2019). Haematological, Blood Biochemical and Immunological Responses to Gradual Acclimation to Low-Salinity Water in Walton’s Mudskipper Periophthalmus Waltoni Koumans 1941 (Perciformes: Gobiidae). Bulg. J. Vet. Med. 22, 13–25. doi: 10.15547/bjvm.2021
Sparks J. S. (2008). Phylogeny of the Cichlid Subfamily Etroplinae and Taxonomic Revision of the Malagasy Cichlid Genus Paretroplus (Teleostei: Cichlidae). Bull. Am. Mus. Nat. Hist. 314, 1–151. doi: 10.1206/314.1
Sundell E., Morgenroth D., Ekström A., Brijs J., Axelsson M., Gräns A., et al. (2021). Energetic Savings and Cardiovascular Dynamics of a Marine Euryhaline Fsh (Myoxocephalus Scorpius) in Reduced Salinity. J. Comp. Physiol. B. 191, 301–311. doi: 10.1007/s00360-020-01336-8
Taki Y. (1978). An Analytical Study of the Fish Fauna of the Mekong Basin as a Biological Production System in Nature. Res. Ins Evo. Bio. 67-72. special publications No.1, Tokyo.
Ulloa P. E., Iturra P., Neira R., Araneda C. (2011). Zebrafish as a Model Organism for Nutrition and Growth: Towards Comparative Studies of Nutritional Genomics Applied to Aquacultured Fishes. Rev. Fish Biol. Fish. 21 (4), 649–666. doi: 10.1007/s11160-011-9203-0
Virabhadrachari V. (1961). Structural Changes in the Gills, Intestine, and Kidney of Etroplus Maculatus (Teleostei) Adapted to Different Salinities. J. Cell Sci. 3 (59), 361–369. doi: 10.1242/jcs.s3-102.59.361
Ward R. D., Zemlak T. S., Innes B. H., Last P. R., Hebert P. D. (2005). DNA Barcoding Australia’s Fish Species. Philos. Trans. R. Soc Lond. B Biol. Sci. 360 (1462), 1847–1857. doi: 10.1098/rstb.2005.1716
Whitney J. E., Al-Chokhachy R., Bunnell D. B., Caldwell C. A., Cooke S. J., Eliason E. J., et al. (2016). Physiological Basis of Climate Change Impacts on North American Inland Fishes. Fisheries 41 (7), 332–345. doi: 10.1080/03632415.2016.1186656
Keywords: marine fish, breeding, orange chromide, salinity, euryhaline fish model
Citation: Padinhate Purayil SB, Thomas SM, Anirudhan A, Sidhick JN, Kandiyil AP, Ebeneezar S, Ignatius B and Achamveetil G (2022) Orange Chromide, Pseudetroplus maculatus (Bloch., 1795): A Potential Euryhaline Fish Model to Evaluate Climate Change Adaptations in Fishes. Front. Mar. Sci. 9:906491. doi: 10.3389/fmars.2022.906491
Received: 29 March 2022; Accepted: 24 May 2022;
Published: 22 July 2022.
Edited by:
Alaa El-Din Hamid Sayed, Assiut University, EgyptReviewed by:
Ercüment Genç, Ankara University, TurkeyJitendra Kumar Sundaray, Indian Council of Agricultural Research, India
Copyright © 2022 Padinhate Purayil, Thomas, Anirudhan, Sidhick, Kandiyil, Ebeneezar, Ignatius and Achamveetil. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suresh Babu Padinhate Purayil, sbabukkd@rediffmail.com
 Suresh Babu Padinhate Purayil
Suresh Babu Padinhate Purayil Shilta M. Thomas2
Shilta M. Thomas2  Jeena Nikarthil Sidhick
Jeena Nikarthil Sidhick Sanal Ebeneezar
Sanal Ebeneezar Boby Ignatius
Boby Ignatius Gopalakrishnan Achamveetil
Gopalakrishnan Achamveetil