Issues on the inclusion of Puntius denisonii (Day), a freshwater ornamental fish of global value, as Schedule-I species under the Wild Life (Protection) Amendment Act, 2021 of India
- 1Ornamental Fisheries Training, and Research Institute, Udaipur, Rajasthan, India
- 2Centre for Aquaculture, and Aquatic Animal Health Management, Kerala University of Fisheries, and Ocean Studies, Kochi, Kerala, India
Puntius denisonii is popularly known as Miss Kerala in India or Denison barb or Red line torpedo barb in the global ornamental fish trade. The species is endemic to fast-flowing rivers and streams of the Western Ghats of India. The species was not very popular earlier in aquatic trade but has been in great demand in global aquarium trade since it was exhibited at AQARAMA 1997 in Singapore and ranked third under the new species category. The export of the species from India started in 1996–1997, which increased progressively and constituted about 60%–65% of a total of 1.44 million US$ worth of ornamental fish exported from India in 2007–2008. Thereafter, it started declining and presently became negligible. It was attributed to depleting stocks of P. denisonii in rivers and streams of Western Ghats. The species was recommended to be listed as endangered on the IUCN red list in a CAMP workshop held at NBFGR, Lucknow, India in September 1997, owing to habitat degradation and the declining number of mature individuals in the wild. It was categorized as Vulnerable in 2009 and Endangered in 2015 under the IUCN red list. The Department of Fisheries, Government of Kerala has restricted the collection of smaller size fish from natural water bodies since 2008 to revive wild stocks. The Ministry of Environment, Forest and Climate Change, Government of India has now proposed to include P. denisonii along with two other freshwater fish species, Semiplotus semiplotus (Assamese kingfish) and Osteobrama belangeri (Manipur osteobrama), as Schedule-I species under the Wild Life (Protection) Amendment Act, 2021 of India. The species listed under this Schedule are prohibited to be hunted throughout the country. The captive breeding technology of P. denisonii has already been developed in the country more than a decade back, and fish is being produced commercially at several farms presently including hatcheries of the Kerala Government. The species is also being cultured and produced on a commercial scale by many ornamental fish farmers of Indonesia and supplied to the global ornamental fish trade at cheaper rates, and more color strains. The major factors that are responsible for the depletion of the stocks of P. denisonii and the overall fish biodiversity of Western Ghat regions are discussed in detail. The conflicts and repercussions that will arise because of the inclusion of Denison Barb or any other freshwater fish as Schedule-I species under the Wild Life (Protection) Amendment Act, 2021 of India are also discussed.
Introduction
A senior contributing editor of AMAZONAS magazine, Red Talbot, published an article on the 28 June 2013 issue of the magazine titled “India’s underground fish trade” (Talbot, 2013). The article begins with a statement that activists have been advocating a complete ban on marine aquarium fish collection, and regularly attack the marine aquarium trade, but the same level of debate does not occur among freshwater aquarists. The writing of Red Talbot was based on the article “Uncovering an obscure trade: Threatened freshwater fishes, and aquarium pet markets” published in the journal Biological Conservation (Vol. 164, Year 2013), authored and lead by Rajeev Raghavan, a fisheries science researcher and conservation biologist from India. Both these articles emphasized that India’s freshwater aquarium trade is having a detrimental impact on endangered, highly endemic fishes frequently harvested, and exported for aquarium usage. The species in question was Puntius denisonii (Day, 1865) a freshwater ornamental fish, endemic to the Western Ghats (WGs) of India. The article of Red Talbot not only initiated a debate among freshwater aquarists as he desired, but also, after 8 years, resulted in the listing of P. denisonii as Schedule-I species under the Wild Life (Protection) Amendment Act, 2021 proposed for implementation by the Ministry of Environment, Forest, and Climate Change (MOEF & CC), Government of India (GOI), vide Bill number 159. The Bill (Bill No. 159, 2021) seeks to increase the species protected under the law, and implement the Convention on International Trade in Endangered Species (CITES) of wild fauna and flora. CITES is an international agreement between governments of member countries to ensure that international trade in specimens of wild animals and plants does not threaten the survival of the species. Any fauna once listed under Schedule-I is prohibited to be hunted throughout the country except under threat to human life or in case of a disease that is beyond recovery, and a person is liable to the harshest penalties for violation of the law. The action of MOEF & CC has raised many questions that need to be answered and analyzed.
i. Did it require such a stringent measure on the part of MOEF & CC, GOI to conserve wild stock of P. denisonii?
ii. Why was only P. denisonii included as Schedule-I species, and no other native freshwater ornamental fish species exported from India that are categorized threatened and included in the IUCN red list?
iii. Is it the aquarium trade that is responsible for the depletion of wild stock of P. denisonii, and many other endemic freshwater ornamental fish species of WGs?
iv. If it was not the aquarium trade, then what were the factors for the depletion of wild stocks of P. denisonii, and other endemic fish species?
There is a need to review, if it is justified or unjustified. However, it will require the readers to know the physiography of WGs of India, and its fish biodiversity, as well as the importance of P. denisonii, and other endemic species in the domestic and global aquarium trade. The major contributing factors that are causing the depletion of fish biodiversity in the WGs are also elaborated and discussed.
Western Ghats: World heritage site
The WGs of India is a UNESCO world heritage site. The WGs cover an area of about 129,037 km2 stretching to a length of 1,490 km along the western coast of India from Tapi Valley in the north (about 21°C16’ N) to Kanyakumari in the south (8°C19’ N), traversing through six coastal states of the country, viz., Gujarat, Maharashtra, Goa, Kerala, Karnataka, and Tamil Nadu (Gadgil, 2011). The WGs along with its geographical extension in the wet zone of Sri Lanka is known as one of the eighth “hottest hot spots” of biodiversity in the world (Myers et al., 2000), second only to the Eastern Himalaya, and one among the four biodiversity hotspots in India. The WGs covers about 5% of the total land of India, and harbors nearly 4,000 species of flowering plants, which are about 27% of the country’s total plant species, about 645 species of evergreen trees including 56% endemic to the Ghats, and 850–1,000 species of the bryophytes including 682 species of mosses with 28% endemism. A total of about 350 (20% endemic) species of ants, 330 (11% endemic) species of butterflies, 174 (40% endemic) species of donates (dragonflies and damselflies), and 269 (76% endemic) species of molluscs (land snails) have been described from this region. The range is home to 120 species of mammals, 225 species of reptiles, 220 (78% endemic) species of amphibians, and 508 species of birds, which include many endemic to WGs. This diversity has been in continuous decline over the last century and more especially in recent decades, with many biological communities and types being almost eliminated due to the ever-increasing human activities and climate change (Gadgil, 2011). A large number of streams that originate from the WGs flow eastward and drain to the east coast in the Bay of Bengal through the Godavari, Cauveri, Krishna, Thamiraparani, and Tungabhadra rivers but a few streams/rivers, viz., Periyar, Bharathappuzha, Pamba, Netravati, Sharavathi, Kali, Mandovi, and Zuari, flow westwards and drain to the west coast in the Arabian Sea. All the west-flowing rivers are fast-moving due to a steeper gradient compared to east-flowing rivers (Gadgil, 2011).
Fish diversity of Western Ghats
The southern and central division of WGs, which includes rivers and streams of Kerala, has been identified as one of the few sites in the world showing exceptional biodiversity and a high degree of endemism with respect to freshwater fishes (Kottelat and Whitten, 1996). Shaji et al. (2000) have comprehensively documented the freshwater fish diversity of WGs and listed 287 fishes, of which 192 (67%) were endemic and 18 were exotic. The list included 106 fish species that were of ornamental importance. Dahanukar et al. (2004) reported the presence of a total of 288 species of fish belonging to 12 orders, 41 families, and 109 genera, of which 118 species were endemic and 51 were unique to WGs. The status of 105 of 288 species was not known due to data deficiency, but among the remaining 183 species, 58 species were categorized as Lower Risk (LR), 41 as Vulnerable (VU), 54 as Endangered (EN), and 24 as Critically Endangered (CR), while the remaining 6 species were exotic. It suggests that at least 41% of fish fauna is threatened by being either VU, EN, or CR. The most species-rich families were the Cyprinids (72 species), hill stream loaches (34 species; including stone loaches, now regarded as a separate family), Bagrid catfishes (19 species), and Sisorid catfishes (12 species).
The number of species was reported to be 290 by Dahanukar et al. (2011) with the addition of two new species, i.e., Mystus seengtee and Channa diplogramma. It included 189 endemic species with 12 species as CR, 54 as EN, and 31 as VU. The number of species was reported to increase to 320 in a new study by Dahanukar and Raghavan (2013) belonging to 11 orders, 35 families, and 112 genera including some secondary freshwater species that can also live in brackish water and marine habitats. The list includes 212 endemic species, of which 47% were categorized as threatened or nearly threatened category. Thereafter, a few more new species have been reported from WGs in the recent past that includes Pethia longicauda (Katwate et al., 2014), Pethia sanjaymoluri (Katwate et al., 2016), Channa pseudomarulius (Britz et al., 2017), Aenigmachanna gollum (Britz et al., 2019a), Channa rara (Britz et al., 2019b), and Waikhomia hira (Katwate et al., 2020). Many of the endemic species of WGs are popular in the global aquarium trade. It mainly includes melon barb, several species of Dawkinsia barbs, zebra loach, Horabagrus catfish, dwarf pufferfish, and dwarf Malabar pufferfish, and most importantly P. denisonii presently known as Sahyadria denisonii (Raghavan et al., 2013a). There is a higher fish richness in the southern part of the WGs than in the northern and the highest in the Chalakudy River alone with 98 species (Raghavan et al., 2008). The other rivers with high species numbers include the Periyar, Bharatapuzha, Pamba, and Chaliyar, as well as upstream tributaries of the Kaveri, Pambar, Bhavani, and Krishna rivers (Shaji et al., 2000; Shaji and Easa, 2001; Kurup and Radhakrishnan, 2006a, and Kurup and Radhakrishnan, 2006b; Arunkumar and Manimekelan, 2018). Mercy and Malika (2010) reported that out of the 300 odd species of freshwater fishes of the WGs, 155 species have ornamental value.
Red Line Torpedo Barb: Puntius denisonii
The P. denisonii (Day, 1865) named after Sir William Denison, popularly known as Red line torpedo barb (RLTB), is a very popular freshwater ornamental fish in the global aquarium trade. It is called so owing to its torpedo-shaped body, and a deep red-colored line running from the tip of the mouth to the middle of the body. It belongs to the order Cyprinidae and the family Barbinae. RLTBs are represented by two distinct species, denisonii and chalakkudiensis (Pethiyagoda et al., 2012), which are quite different from other similar barbs, and are now placed under a different genus Sahyadria based on osteology and molecular characteristics (Raghavan et al., 2013a). However, in the present communication, it has been referred to as P. denisonii only because of its popularity.
The P. denisonii inhabits fast-flowing rivers and streams of WGs. It is a benthopelagic species that is gregarious, and shoals are known to occur in rocky pools with thick overhanging vegetation along the banks (Radhakrishnan and Kurup, 2005). They thrive in a sub-tropical climate in water with a pH of 6.8–7.8, a water hardness of 5-25 dGH, and a temperature of 65 to 79°F (18 to 26°C). Denison’s barb is an omnivore and feeds on insects, worms, small crustaceans, organic debris, formulated flakes, pellets, and other processed feeds. The average size of the fish is 9–11 cm (3.5–4.3 inches) with a maximum length of 15 cm, and an age range of 5–8 years. A young fish attains sexual maturity at 12 months of age and a total length of 7.8 ± 2.2 cm (Mercy et al., 2015). The breeding technology of the fish has been developed in the country (Mercy et al., 2013 and Mercy et al., 2015) and presently about 1.00 lakh fish are being produced annually at the fish farm of the Department of Fisheries, Kerala and two to three private ornamental fish farms (personal communication of the second author with hatchery owners).
Distribution of P. denisonii in WGs rivers
P. denisonii is endemic to the fast-flowing hill streams and rivers of the southern region of WGs specifically in the state of Kerala, and sparsely in Karnataka as a fragmented population (Biju et.al., 2000; Shaji and Easa, 2001; Mercy et al., 2002; Kurup and Radhakrishnan, 2006b; Prasad et al., 2008, and Raghavan et al., 2010). Talwar and Jhingaran (1991) reported the presence of P. denisonii in the rivers of Alaram (a tributary of Vallapatnam), Chalakudy, Chaliyar, Kallada, and Kallar (Table 1). It was later reported in Chandragiri, Periyar, Vallapatnam (Biju et al., 2000), Pampa (Shaji and Easa, 2001), Achenovil (Shaji and Easa, 2001), Bharatapuzha (Mercy et al., 2002), Manimala (Thomas, 2010), Karingode (Kurup and Radhakrishna, 2006b), and Anjarkandy (Mercy and Malika, 2010).
The presence of P. denisonii has been reported in 15 out of the 44 rivers flowing through the state of Kerala, and originating in WGs (Table 2). The Kallar River is not counted separately as it is a tributary of the Pampa or Pamba River. All these rivers are west-flowing except for the Bhavani River that originates in the Nilgiri hills of Tamil Nādu. Thampy et al. (2021) have reported the presence of P. denisonii in the Wayanad part of the Kabini river. It is the first incidence of the presence of this fish in any east-flowing river. The rivers of WGs are small in terms of length and width, with low water discharge, and are entirely monsoon fed, which either completely dry out during summer or shrink into a small rivulet. These rivers are more prone to environmental effects as they are small and also lack a delta (Latha and Vasudevan, 2016). A fast flow owing to hilly terrain, high elevation, meandering curves, and large riparian zones are important characteristics of all these rivers. All of these rivers originate 1,000 m above sea level except for Achenovil, Anjarkandy, and Manimala. A few of these rivers, viz., Bharthapuzha, Chaliyar, Pampa, Periyar, and Vallapatnam, are fed by several tributaries, while others have either one or none. Several dams have been constructed on a few of these rivers for irrigation, electricity generation, or drinking water purposes.
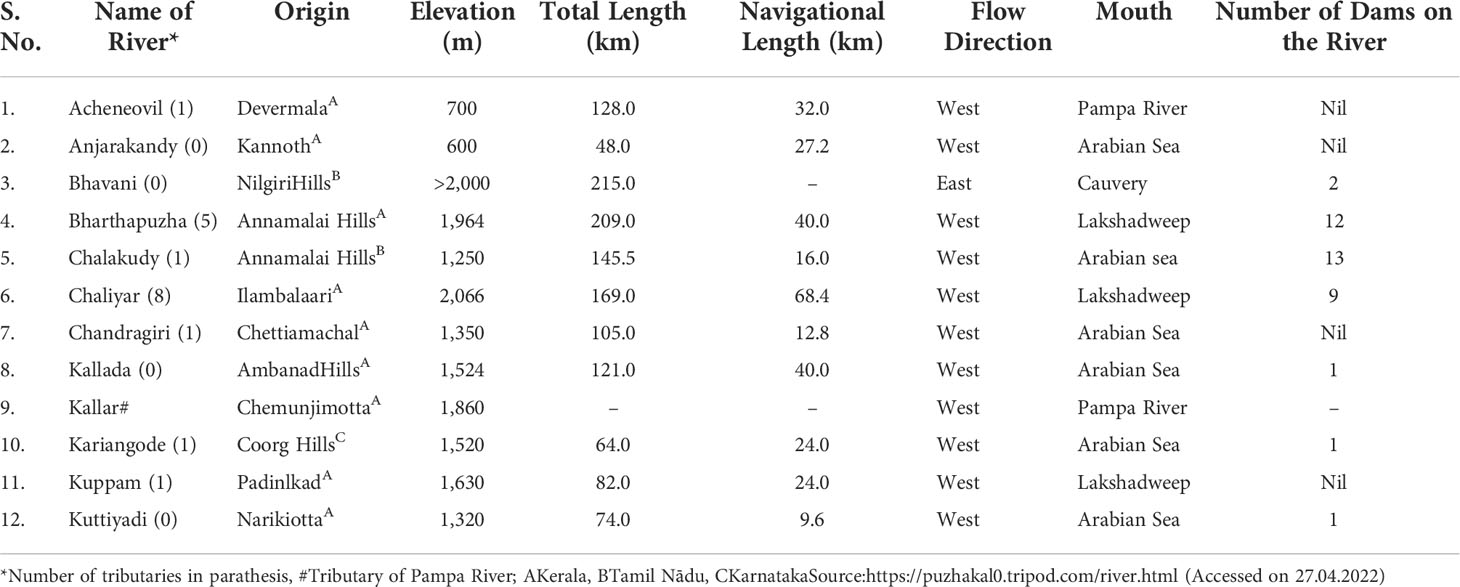
Table 2 Physiographic characteristics of the Western Ghats Rivers known for the presence of P. denisonii during the period 1991 to 2022.
Export of ornamental fishes from India and the importance of P. denisonii in the global aquarium trade
The export of freshwater ornamental fish from India is reported to begin in the year 1969, and the first consignment was valued at Rs. 16.40 lakhs only (Sekharan and Ramachandran, 2006). However, the contribution of India to the global export of ornamental fishes remains very low (Figure 1). It was only 1.558 million US$, i.e., Rs. 10.884 crores (MPEDA), out of the global export of 322 million US$, i.e., 0.48% of the total world export during 2019–2020 (Trend Economy, 2020).
India is known in the global aquarium trade for the wild-caught indigenous fishes mainly from rivers, streams of northeast states, and WGs, which constitute about 85%–90% of total ornamental fish export (Silas et al., 2011; Jayalal and Ramachandran, 2013). It initially included several species of Channa, Botia, Puntius, Barilius, Garra, Glyptothorax, Horabagrus, Schistura, etc. (Silas et al., 2011). P. denisonii was not known in the international market before it was first collected and exported to Germany in 1996 (Raghavan et al., 2007). Subsequently, it was exhibited at “AQUARAMA 1997 Singapore”, an international exhibition on ornamental fishes, under the “New Species Category”, and won the third prize (Anon, 2006). Thereafter, it soon became a popular aquarium fish among international aquarium hobbyists owing to its vibrant red color marking along the lateral line in the anterior half and its torpedo-shaped body. It constituted about 60%–65% of the total live ornamental fish exported from India during 2007–2008, which was valued at 1.44 million US$ (Mittal, 2009). About 1.5 lakh individual fishes were exported by the Kerala Aquatic Ventures Private Limited in 2010–2011 (Raghavan et al., 2012) while over 3.00 lakh RLTBs were reported to be exported from India during the period 2005–2012 to seven countries (Raghavan et al., 2013b). These numbers could be much higher as other fish that were exported under the generic label “Live ornamental fish” possibly included RLTBs (Raghavan et al., 2013b). It was also estimated that about 5,000 P. denisonii were being collected and exported from Kerala on a weekly basis, which may include 1000–1750 females (Raghavan et al., 2012).
The total value of ornamental fish exported from India started to decline from 2007–2008 onwards (Figure 1), and the same trend continued up to 2014–2015 (0.933 million US$; Rs. 5.66 crores). It was possibly because the share of P. denisonii reduced from export consignments of India as the Kerala fisheries department imposed a restriction on its collection from the wild during 2008. It was the same period when captive-bred P. denisonii from other countries was available for the global aquarium trade (Mittal, 2009). The export value started to increase from 2014–2015 onwards, and achieved the highest value in 2019–2020 (1.55 million US$; Rs. 10.84 crores), and expected to be more than double during 2021–2022, and further higher during 2022–2023 (personal communication of the first author with some exporters). It is because of the increasing demand for pond-reared Koi carps and livebearers from India in United Arab Emirates. The major share of native species from India is presently composed of Botia lohachata (Botia loach), Botia striata (Zebra loach), Waikhomia sahyadriensis (Maharaja barb), Channa aurantimaculata (Spotted snakehead), Channa barca (Barca snakehead), Channa bleheri (Rainbow snakehead), Etroplus canarensis (Canara pearl spot), Tor putitora (Golden Mahaser), Hemibagarus kudremukhensis (Nilgiri mystus), and a few more.
Inclusion of P. denisonii in the IUCN red list
The workshop on “Conservation Assessment and Management Plan (CAMP)” held at the National Bureau of Fish Genetics Resources (NBFGR), Lucknow, India in September 1997 recommended the inclusion of P. denisonii under the endangered category. A total of 329 freshwater fish species from Indian waters were assessed in the workshop, of which 47 were categorized as critically endangered, and 98 as endangered. The RLTB, P. denisonii, was one of the endangered categories (Molur and Walker, 1998). It was recorded to be present only at four locations in the WGs section of Kerala, viz., Cheenkannipuzha, Mundakayam (Mundayapuzha river), Achankovil, and Chaliyar, with a distribution range of <5,000 km2. Both Cheenkannipuzha and Mundayapuzha are tributaries of the river Vallapatnam, i.e., one of the 41 west-flowing rivers of WGs in Kerala. The other parameters that supported the categorization of P. denisonii as endangered were loss of habitat due to poisoning, pollution, and local trade as well as unavailability of captive breeding technology. The fish was categorized as VU in 2009 and EN in 2015 under the IUCN red list (Ali et al., 2015).
Is aquarium trade responsible for the depletion of P. denisonii wild stock?
The aquarium trade has been held responsible for the depletion of wild stock of P. denisonii or many more indigenous fishes of the northeast and WGs of India (Dahanukar et al., 2004; Kurup et al., 2004; Sekharan and Ramachandran, 2006; Raghavan et al., 2007; Daniels, 2011; Dahanukar and Raghavan, 2013; Jayalal and Ramachandran, 2013; Raghavan et al., 2013b), which have been included in the IUCN red list. These indigenous fishes were not in much demand in the domestic aquarium trade until very recently, but the demand increased with the growing interest among hobbyists for planted and biotope aquarium setups. Presently, few species of the barbs, viz., Puntius filamentosus, P. denisonii, and P. ticto, and a few hill stream loaches, i.e., B. striata (Zebra loach), Botia dario (Bengal loach), etc., are in demand in the domestic aquarium trade but the numbers are very few (personal observation of the first author). The native fish are mainly collected for export purposes only. Are these collected in such a large number that it will lead to their stock depletion or habitat destruction and will eventually become endangered or critically endangered? The answer is “NO”. The number of fish that are collected and exported (Raghavan et al., 2012 and Raghavan et al., 2013b) is insignificant to the number and quantity of fish harvested for food purposes (Mohite and Samant, 2013; Shaji and Laladhas, 2013). The natural stocks of many of the fish species, including both edible and ornamental, have depleted due to various reasons that remain undescribed while blaming the aquarium trade. The major contributing factors are as follows:
Distribution and stock assessment
The information on the distribution of P. denisonii and its population in rivers and streams of WGs is possibly incomplete even at present. The fish was introduced to the global aquarium trade in 1997 (Anon, 2006) while it was reported to be endangered in the CAMP workshop held at NBFGR Lucknow (India) during September 1997 (Molur and Walker, 1998). It was known to be present only in three rivers, i.e., Achankovil, Chaliyar, and Vallapatnam (Cheenkannipuzha, and Mundayapuzha) at that time. It was only after the year 2000 that the presence of the fish was reported in other rivers of the WGs, which increased to a total of 15 rivers in 2015 (Table 2). It is evident that the presence of fish in more rivers of WGs was known only after it was introduced to the export trade. However, it was also possible that the stock of the fish has already depleted in those rivers also before its presence was reported. The increasing export demand would have definitely resulted in an increased collection of “Live Fish” from these rivers. However, it should also be noted that presently there is not much demand for wild-collected P. denisonii from India in the global aquarium trade as fish breeders in other countries are breeding the fish in captivity (Mittal, 2009) with more varieties of new strains that have a higher demand in the global trade. There is a need for studies to find out if the reduced collection of “Live fish” has resulted in the revival of stocks in rivers of WGs.
There are only a few studies on the stock assessment of P. denisonii in the rivers of WGs (Kurup and Harikrishnan, 2008; Raghavan et al., 2008; Mercy and Malika, 2010; Dahanukar et al., 2011). Kurup and Harikrishnan (2008) studied the population characteristics and stock assessment of P. denisonii from different locations of Kanjirpuzha River, which is a major tributary of the Bharathapuzha River on a bi-monthly basis during the period 2001–2003. The growth parameters, total mortality, natural mortality, and MSY were calculated based on the catch data. The potential yield, current annual yield, and MSY of P. denisonii were estimated to be 1.52, 1.036, and 0.94 tons, respectively. It was concluded that the population of P. denisonii is not under threat from overfishing, and the Kanjirampuzha River supports a sustainable population.
Mercy and Malika (2010) conducted a survey of 11 rivers of WGs, viz., Chandragiri, Kariangode, Kuppam, Valapattnam, Anjarkandipuzha, Kuttiyadi, Chaliyar, Chalakudy, Bharathapuzha, Bhavani, and Pamba, for the period 2003–2007. It was concluded that P. denisonii was not endangered, and the stocks were not depleted in nature except in the Chalakudy River, though the collection of the fish has increased considerably from earlier studies. In a further study from October 2008 to December 2009, the total biomass was estimated to be only 33% of the virgin stock in the Valapattanam and Karingode rivers. The reasons for stock depletion were stated to be habitat degradation due to unethical practices of fishing like the use of dynamites, copper sulfate, and other poisons that are employed for harvesting food fishes but it ultimately affects non-target groups like ornamental fishes.
Obstruction to the rhythmic flow of rivers
The natural flow of streams and rivers is essential in determining the biodiversity richness of riverine flora and fauna. A number of species and their life cycle are known to have evolved following the rhythmic flow of the rivers (Dynesius and Nilsso, 1994). The natural and rhythmic flow of many rivers around the world has been obstructed due to the construction of a large number of multipurpose dams (Dudgeon, 2000; Pringle et al., 2000; Nilsson et al., 2005) including those of WGs. The construction of dams converts a river into chains of reservoirs with no flow in between the reservoirs. The Periyar river in between Parambikulam, Sholayar, Mullaperiyar, and Idukki dams does not flow for long before it is joined by the next tributary or recharged from the catchment area (Latha and Vasudevan, 2016). Studies indicate that the flow diversion, alteration, and reduction due to dams have a significant impact on the riverine ecosystem, its microhabitats, and flora and fauna in several ways (Bartens et al., 2008). There are 80 dams in the rivers of Kerala along the length of WGs, of which 43 are large dams. The WG dams and Small Hydro Projects (SHP) account for about 80% of India’s hydropower generation (Jumani et al., 2018). These dams and SHPs have significantly altered the physiochemical characteristics, blocked sedimentation and nutrient transport, obstructed fish migration, and encouraged invasions of alien fish species by disrupting the longitudinal, lateral, and vertical connectivity of rivers (Dudgeon, 2000; Rosenberg et al., 2000).
The fishery of Tenualosa ilisha, an anadromous Indian shad, collapsed in the river Cauvery above the Mettur Dam, Tamil Nadu as the fish from Cauvery-Coleroon system could not ascend the river Cauvery for breeding. The construction of three lower barrages, i.e., the Upper Anicut, the Grand Anicut, and the Lower Anicut, partly restricted the upstream migration of T. ilisha, but it was reported to have completely disappeared from the upstream stretches after the commissioning of Mettur Dam (Sugunan, 1995).
The fish community responses to streamflow alterations and habitat modification by small SHPs in Netravati River of WGs were studied by Jumani et al. (2018). The fish assemblages were surveyed in two dammed tributaries and one undammed tributary of the river at different locations including upstream and downstream. It was found that the undammed river had high species richness, diversity, and endemicity compared to the two dammed rivers. The percentage of adults and juveniles of rheophilic species (Tor khudree) was higher in the undammed river while dammed rivers were dominated by eurytopic species Devario malabaricus. The rivers of south India are known for the presence of rheophilic species (Sudasinghe et al., 2021).
Loss of riparian zone and vegetation
The riparian zone is the transition zone between the terrestrial and aquatic habitat, and the vegetation in this zone is known as riparian vegetation. The vegetation in the riparian zone is highly influenced by the quantum and flow of water in the river channel. The altitude, climatic conditions, and soil characteristics determine the nature of plant communities (Nair, 1994) while the physical and the geomorphological features of the watershed are also influenced by riparian vegetation, which characterizes the riparian forest dynamics (Naiman et al., 1998). A reduced or increased flow of river may severely influence the abundance, and reduction in the population rate of the specific species in the riparian vegetation (Tabacchi et al., 1996; Braatne et al., 2008). The riparian canopy regulates water temperature in the streams/rivers by shadowing them, which, in turn, plays an important role in the distribution of fish communities (Marsh-Matthews and Matthews, 2000). It also controls stream sediments, and nutrient dynamics by supplying organic matter via litter fall, while their root systems stabilize the bank and filter lateral sediment and nutrient inputs (Naiman and Decamps, 1997). The submerged leaves’ surfaces are sites of primary and secondary production by microalgae, bacteria, and planktonic microorganisms (Dosskey et al., 2010). A mapping of a stretch of the Chalakudy River showed that 60 km of riparian vegetation has submerged due to the construction of six dams and dried up along 28.8-km stream/river banks due to the complete diversion of waters downstream to dams (Hyder, 2003; Hyder and Devika, 2020). It has resulted in the destruction of about 2.66 km2 of riparian forests, which is equivalent to 80% of the presently remaining riparian vegetation in the Chalakudy River. Although no direct studies have been conducted on the effect of the loss of riparian vegetation on the depletion of P. denisonii, it could be one of the important factors of fish biodiversity depletion in WGs (Hyder, 2003; Mohite and Samant, 2013; Jumani et al., 2018).
Sand mining in WG rivers
The river beds are characterized by sediments, i.e., loose sand, clay, silt, and other soil particles that settle at the bottom. These sediment beds largely affect the water quality and biological productivity of a river or stream by controlling light penetration and influencing ecological functioning (Owens et al., 2005). These sediments are breeding and grazing ground for many freshwaters and estuarine fishes; therefore, any change in the quality and quantity of sediments influences fisheries both upstream and downstream (Brown et.al., 1998; Harvey and Lisle, 1998; Paukert et al., 2008; Freedman et al., 2013; Mingist and Gebremedhin, 2016). The sand mining from rivers around the world including WGs rivers has resulted in irreversible losses (Koehnken and Rintoul 2018). It causes channel instability both up- and downstream, disrupting existing equilibrium and erosion of banks due to incision. Aquatic ecology including the presence of riparian vegetation is significantly dependent on the sand deposits in the river bed as sand deposits help maintain a high water table in rivers. The local fish populations were found to be affected in the Arno-Garno and Ribo rivers in Ethiopia due to sand mining that destroyed spawning grounds, interfered with migratory routes, and caused large-scale fish kills (Mingist and Gebremedhin, 2016). The replacement of riffles with pools and decreased flow velocity due to sand mining were found to replace lotic species with lentic and invasive species (Brown et al., 1998; Harvey and Lisle, 1998; Paukert et al., 2008; Freedman et al., 2013).
Wyżga et al. (2009) studied the impact of sand mining and channelization on the fish communities of the Czarny Dunajec River in the Polish Carpathians. The diversity and abundance of fish species were significantly higher in multi-channeled unmanaged rivers without any sand mining practices than in single channels with incised cross-sections due to mining. Freedman et al. (2013) observed that the spawning of brood-hider and substratum chooser fish species was more adversely impacted than nest or open substratum spawners due to sand mining. Heavy siltation of streams due to deforestation and sand mining, which modify the stream beds, directly affects several endemic species as it degrades their breeding substrates (Dahanukar et al., 2011). Hill stream loaches of the families Balitoridae, Cobitidae, Nemacheilidae, and several species of cyprinids including the critically endangered Neolissochilus wynaadensis and Tor remadevii are particularly vulnerable to siltation.
Sand mining is a very common practice in almost all the rivers and streams of WGs despite restrictions by the government through the enactment of the Kerala River Bank Protection and Sand Removal Regulation Act 2001 (Hyder, 2003). The sand beds of the Bharatapuzha River are replaced by grasses and sedges due to the ever-increasing sand mining over the last three decades (Latha and Vasudevan, 2016). It was estimated that about 2.856 × 106 tons of sand is extracted from the Bharatapuzha river annually. It is almost 54.92 times more than the annual replenishment of 0.052 × 106 tons. The river bed is deepened by more than 20 ft in many places. The river bed remains exposed for most of the time during the year, and the river fails to flow full even during the monsoon. The sand deposits are reported to be completely lost from the Chalakudy River bed. About 0.92 × 106 tons of sand was extracted from the river annually, which is 76.66 times more than the annual replenishment of 0.012 × 106 tons. The river bed has been lowered by an average of 6–8 m in many places (Latha and Vasudevan, 2016). The river bed of the Pamba River has been lowered to about 1.23 m during 1985–2000 (8 cm/year). This is attributed to the heavy extent of sand mining upstream as well as downstream. About 0.84 × 106 tons of sand is annually extracted from the Pamba River bed, which is 30 times the annual replenishment rate of 0.028 × 106 tons (Latha and Vasudevan, 2016).
Pollution of Western Ghats rivers
The ever-increasing urbanization, industrialization, agricultural activities, etc. have been a cause of water pollution around the globe, and adversely affected fish diversity and production (Shelford, 1918; Jones, 1964; Bukola et al., 2015; Silva et al., 2016; Malik et al., 2020). The completely pristine water of WGs rivers is also being constantly polluted by the discharge of several large industries built in the catchment area of the river banks. The discharge from the Gwalior rayons factory by Grasim industries in Mavoor located in the Chaliyar catchment was reported to result in heavy fish mortality and dislocation of Nereid worms in the Chaliyar River flowing between Elamaram and Pallikkadavu near Calicut in a study during 1979 by the Central Marine Fisheries Research Institute (Latha and Vasudevan, 2016). Industrial pollution was observed to pose a serious threat to the riverine ecosystem in the lower reaches of Periyar about 15 km upstream from the backwaters of Cochin. About 250 small and big industries engaged in the production of fertilizers and pesticides, petroleum refining, heavy metal processing, radioactive mineral processing, rubber processing, tallow extraction, battery manufacturing, mercury production, acid manufacturing, and pigment and latex production are concentrated between Angamaly and Cochin along the river bank. The industries of the Edayar-Eloor area consume about 190,000 m3 of water per day and discharge 75% of the same wastewater along with a variety of pollutants. Greenpeace India describes the lower Periyar as a “Global Toxic Hotspot”, with alarming levels of hazardous chemicals such as ammonia, DDT, trivalent chromium, cyanide, endosulfan, and BHC (Latha and Vasudevan, 2016).
Land-use changes
The land uses have changed many folds in the catchment area of WGs rivers due to the ever-increasing population, urbanization, industrialization, and livelihood earning activities. There has been an upsurge in construction in the lower catchment region of the Pamba River owing to sand mining and a large-scale livelihood shift from agriculture. As a result, the Vembanad backwaters that receive drainage from the Pamba River have shrunk by almost 12,000 ha, and biodiversity is depleted. The Varaalchal wetland in Koyipram Panchayat of Pathanamthitta district of Kerala, once a rich fish resource, and an extensive paddy cultivable wetland of about 500 acres, has become a mere stagnant area of weeds and sewage due to various commercial exploitation activities (Latha and Vasudevan, 2016). The Aranmula wetlands where paddy was grown traditionally has become waterlogged due to a check dam across the Valiyathodu stream, which was a natural flood escape route between the Pamba and Aranmula wetlands. The paddy cultivation had reduced by 50% in the year 2000. A large part of the forest area in the Periyar River basin has been cleared for various developmental activities. Land use and land cover study of the Bharathapuzha River basin conducted using imageries from 1973 to 2005 revealed that there was 31% depletion in the natural vegetation cover and 8.7% depletion in wetland agriculture area. The urban spread in the basin had increased by 32% (Latha and Vasudevan, 2016).
A large area in Vazhachal Forest Division (Chalakudy) has been converted into forest plantations for timber as part of the forest department policy. The Plantation Corporation of Kerala has converted large tracts of the riparian forest into oil palm and rubber plantations. Deforestation in the upper catchments had an impact on river flow that causes many of the streams to dry up in the summer months. Good riparian vegetation has become rare in the lower zones of Chalakudy due to high disturbance caused by anthropogenic activities.
The paddy fields during flooding are highly suitable grounds for the breeding of many species of fish (Shaji and Laladhas, 2013). The total extent of paddy fields in the Kerala state was 880,000 ha in 1970–1971, and rice accounted for the highest share (32%) of the total cropped area in the state (Athira and Kumar, 2016). It reduced to 560,000 ha in 1990–1991 and 230,000 in 2007–2008 (Thomas, 2011). The total area of paddy fields in the state was only 202,907 in 2018–2019. The loss of forest in the WGs has been so rapid that out of the original 182,500 km2 of primary vegetation, only 12,450 km2, i.e., 6.8%, is available (Myers et al., 2000; Gunawardene et al., 2007). All these changes in land-use patterns have been responsible for habitat destruction and fish biodiversity depletion.
Invasion by alien fish species
A species could be alien to a new habitat if introduced not only from a distant geographical location but also from a different habitat in the same region. The invasion of alien species has been a major cause of depletion of endemic fish species all around the globe (Canonico et al., 2005; Burrill, 2014; Erarto and Getahun, 2020) including in India (Joshi et al., 2014; Joshi et al., 2021), which has reduced fish production as well as aquatic biodiversity. The rivers and reservoirs of WGs are no exception to this (Sugunan, 1995; Radhakrishnan et al., 2012; Biju Kumar et al., 2015; Thampy et al., 2021). The invasion of alien species has not only adversely affected minor or marginal fisheries but even major commercial fisheries. The catches of Cirrhinus cirrhosa were found to be severely declined in Bhavanisagar, Stanley, and other reservoirs of Tamil Nādu after the introduction of C. mrigala, another Indian Major Carp native to the Gangetic River systems of India (Sugunan, 1995). The Mozambique tilapia Oreochromis mossambicus has been the most successful among all the alien fishes. At least 24 reservoirs in Tamil Nadu have tilapia either as the major component of fish catch or represent a dominant fishery. It outnumbers other fishes by 90 to 10 in the Amaravathy reservoir. The Mahseers, once a dominating fishery composed of Tor, T. putitora, and Acrossocheilus hexagonolepis, have also become threatened in many reservoirs of Tamil Nadu (Sugunan, 1995).
Radhakrishnan et al. (2012) conducted an extensive survey covering 465 locations of 25 river systems in WGs from 2000 to 2004. A total of 32 alien species belonging to seven orders and 10 families, mainly Cyprinidae (14), Poeciliidae (4), and Channidae (3), were recorded. The freshwater garfish Xenentodon cancila, snakehead murrel Channa striata, and Mozambique tilapia Oreochromis mossambicus were found to dominate other species in many rivers and locations including pools, runs, riffles, and reservoirs. All the above-mentioned three species are omnivores and known to feed on eggs and young ones of other fish species.
Fish-eating habits of locals
Fish is an integral part of the diet of a Keralite family (Kerala being an 85% fish-eating population; Shyam et al., 2015; Shyam, 2020); one meal features fish every day (Gulati, 1984). The fish consumption in the state of Kerala is 19.41 kg/head, which is the second highest in the country after Tripura with 29.29 kg/head. The all-India average is 9.00 kg/head only (Anon, 2020). A recent study by Shyam (2020) revealed that the monthly mean consumption of fish in three districts of Kerala, i.e., Thiruvanthapuram, Kochi, and Kozhikode, was 9.26 kg per household, which included 6.00 kg of low-value fish. The total fishermen population in the state is 10.44 lakhs (Inland: 23%; Marine: 67%), which is 2.92% of the total population of the state. Fishing is the full-time activity of 152,708 fishermen in inland regions (Anon, 2020). The fishermen population per district in Kerala is 74,597, which was the fourth highest in the country behind Bihar (158,615), West Bengal (140,707), and Andhra Pradesh (115,129). Hence, fishing is a very common activity for rural people inhabiting near the river banks. The fishing activity for many of the locals in rivers of WGs was earlier limited to family consumption only but gradually it also became a source of livelihood, which increased pressure on the fishing (Mohite and Samant, 2013). The conservation of fish biodiversity or selective fishing is never the concern of these local fishing populations probably due to a lack of awareness. Eating dry fish is very popular in many regions of India. The largest dry fish market in Asia is situated at Jagiroad in the Marigaon district of Assam. Several tons of dry fish, both freshwater and marine, are sold here every day. The freshwater fishes that are dried mainly include small-size fishes.
Un-eco-friendly fishing practices
The gill net, cast net, and drag net are few of the common fishing methods in rivers and reservoirs of WGs that are widely used. Each method has its advantages and disadvantages, which are possibly known to all concerned, but few of the other fishing practices employed in rivers of WGs that are serious threats to fish biodiversity as well as fish production are discussed here. It includes Monsoon Floodplain Fishery (MFF), electric fishing, and the use of dynamites.
The MFF or Oothapiduthamis, is the traditional practice of fishing at the onset of the southwest monsoon when freshwater fishes migrate to paddy fields from adjoining rivers and canals for egg-laying. These upward migrating brood fishes are collected by using diverse types of traditional fishing gears (Nathoodu, Adichil, Chaattom, Ottal, Koodu, Kuthuvala, etc.) by fisherfolks and even non-conventional fishermen. Oothapidutham is celebrated as a festival throughout the state of Kerala. Shaji and Laladhas (2013) observed that a total of 1,159 kg of fish was harvested within 6 days from the 489-ha paddy wetland system of Kuzhur-Annamandu Panchayats in Thrissur districts of Kerala. The fishes that were caught included Anabas testudineus, Anguilla bicolor, Channa striata, Amblypharyngodon melettinus, Horabagrus brachysoma, Xenetodon cancilla, Wallago attu, Puntius parrah, P. mahecola, P. filamentosus, Babodes subnasutus, Ompok bimaculatus, Nandus, and Macroganthus guentheri. All the fishes except the species Anguilla bicolor were berried. A few of the important rivers in Thrissur district are Bharatapuzha, Chalakudy, and Chaliyar. The total area under paddy cultivation in the state was 202,907 ha during 2018–2019 as per the Department of Economics and Statistics, Government of Kerala.
The use of dynamites, which is also termed “Fish bombing”, has become a serious problem around the globe (Agnew et al., 2009; FAO & UNEP, 2010; Braulik et al., 2015) including India in both marine and freshwater fishing. It is a fishing practice of using explosives to stun or kill schools of fish for easy collection. It is both an illegal and unethical way of fish collection brought about by the increasing greed of fishermen. Fish bombing kills not only the target fish but also all other unwanted aquatic organisms within the surroundings and destroys the habitat (Saila et al., 1993; Guard and Masaiganah, 1997; McManus et al., 1997; Riegl and Luke, 1999). The use of dynamite fishing has been a common practice in rivers and reservoirs of WGs (Lalmohan and Rama Devi, 2000; Hyder, 2003; Thampy et al., 2021). Hyder (2003) reported counting 37 blasts of dynamite for fishing purposes during 3–4 h walk from Athirapally to Thumboormuzhi in 2001 while surveying riparian vegetation along the middle and lower zones of the Chalakudy River.
Climate change
Global warming and many human-induced activities result in climate change worldwide. India has also experienced a significant increase in temperature except for the Indo-Gangetic plains in the last 60 years, and the country’s average temperature is expected to rise by 4.4°CC at the end of the year 2100 (Nanditha et al., 2020). The summer monsoon rainfall (June to September) over India has also declined by around 6% from 1951 to 2015, with notable decreases over the Indo-Gangetic Plains and the WGs (Kulkarni et al., 2020). The effect of climate change is further pronounced in WGs due to localized changes in the region. The forest cover in WGs has reduced by 2.49% between 2001 and 2016. It has resulted in reduced water retention capacity of the catchment, the conversion of several perennial streams to seasonal streams, and climate change. The mean temperature increased from 30.9°CC in 2001 to 31.9°CC in 2016. All these changes in the climate alter the ecology and, consequently, the biodiversity and hydrologic regime of a region. It affects the sustenance of water that leads to the loss of native biodiversity and the invasion of new species (Ramachandra et al., 2013; Kulkarni et al., 2020). Temperature plays an important role in the growth and development of aquatic animals (Ngoan, 2018; Maulu et al., 2021). A slight change in temperature has a profound effect on the reproduction of fishes by altering the maturation period, breeding season, hatching, and larval development (Pankhurst and Munday, 2011). Extreme climatic events increase the susceptibility of freshwater fishes to infections and disease outbreaks (Lopez et al., 2010). The changing climate was found to accelerate fish diversity in the Kabini river, which experienced a severe shortage of water during the summers of 2017–2019 and massive floods during the monsoons of 2018–2019. The post-flood months of 2018 and 2019 resulted in widespread mortality of fishes at several locations in the Kabini catchment due to bacterial disease outbreaks (Thampy et al., 2021).
Conservation status of fish biodiversity of Western Ghats
The fish biodiversity and the conservation status of fishes of WGs have been studied and reviewed by a number of researchers in the last two decades (Table 3).
The total number of fish species was found to increase due to both the discovery of new endemic species and the increasing presence of alien species in the WGs. However, the endemism, as well as the conservation status reported by different researchers, was observed to be highly varying (Table 3). The reason for these variations is beyond the scope of the present article but needs to be examined. Presently, the issue of concern is to specify that several species of fish from WGs, which include many species of ornamental importance, have been categorized as CR, EN, and VU at different stages, but P. denisonii has been treated indiscriminately and recommended for listing as a Schedule-I species according to Wild Life (Protection) Amendment Act, 2021.
Indiscriminate treatment of P. denisonii
P. denisonii is victimized by its popularity: “popularity” that is probably less in the global aquarium trade and more among scientific communities. Daniels (2011) in his paper “Miss Kerala in Peril” has stated that the species that stayed dormant for 130 years was rediscovered as soon as live specimens and photographs came to light during the 1990s and thereafter it was exhibited in AQUARAMA 1997 at Singapore and ranked third under a new category. “Miss Kerala” became a beauty pageant not only in the aquarium but a common subject of discussion on many national and international scientific platforms with a series of publications in reputed scientific journals with a high impact factor. We encountered more than 100 publications on P. denisonii, very few on biology and culture but many on the threatened status of fish biodiversity of WGs due to the collection of the fish for the aquarium trade. The major factors that have resulted in the depletion of not only P. denisonii but also many other species from both biodiversity hot spots of India (WGs and East-Himalayan regions) that challenged the biodiversity were not discussed. It was truly stated that the “Damsel was in distress” and the “Aquarium trade in India was obscure”, but the damsel was victimized by its fame. Daniels (2011) stated that the species has been unscrupulously caught and traded. It may be appropriate to mention that it was unscrupulously debated and discussed too by scientific communities within the country, which gained the attention of a few researchers outside the country.
Conflicts and repercussions
It was the first time that small-size freshwater fishes like P. denisonii, Semiplotus semiplotus, and Osteobrama belangeri were recommended for listing as Schedule-I of the Wild Life Protection Act of India. O. belangeri was found to be present in the state of Manipur but is presently extinct while S. semiplotus is distributed in the rivers of Assam and P. denisonii is found in many rivers of WGs although stocks may be depleted. Both these fish are caught and consumed by local fishermen as food. There is no fishing method available to exclude these fishes selectively from the catch while fishing in any of the water bodies. The fishing activities and fisheries resources in water bodies of a state are managed by the respective state fisheries departments while any animals protected under Wild Life Protection Act, 1972 are under the control of the local forest department. The state fisheries department will be issuing the license or permission for fishing in these water bodies while the forest department will be arresting the fishermen for fishing as they will be unable to exclude prohibited species from the catch. Fishing in all these water bodies will become a punishable offense for thousands of fishermen and local people collecting fish either for livelihood or food purposes. The implementation of the act may lead to conflicts between different implementing agencies and the local fishermen. It is also expected to face opposition from local fishermen and villagers once they understand the seriousness of the issue. However, it is also doubtful if the concerned agencies could do the same in the field once it is enforced. The Department of Fisheries, Government of Kerala has imposed a ban since 2008 on the collection of P. denisonii smaller than 9–10”. The fish has been part of the export consignment even after that but there was no any recorded evidence if any person was caught (as informed by the second author).
Conclusion
The faunal and floral biodiversity is continuously depleting in many parts of the world mainly due to human-induced activities. A number of international agencies and the governments of respective countries are enforcing several laws (de Klemm and Shine, 1993; UNEP, 2018; Singh and Dukariya, 2021) to protect and conserve the rich biodiversity resources both terrestrial and aquatic. These laws have been highly rewarding in many cases (Vaughan, 2021) but failed to bring desired results with instances of strong opposition by local communities (Muhumuza and Balkwill, 2013). The failures were on account of improper evaluation of merits, demerits, effectiveness, and consequences of the particular law enforcement by the implementing agencies.
The Wild Life Protection Act 1972 of the Government of India and subsequent amendments (1982, 1986, 1991, 1993, 2002, 2006, and 2013) before the year 2021 provided protection to only whale shark (Rhincodon typus), eight species of shark and rays, all the species of sea horses and giant grouper (Epinephelus lanceolatus) among fishes. All these fish species are of very large size with a total body length range of 100–1,700 cm (excluding seahorses) and found in sea only except for Gangetic shark (Glyphis gangeticus), Ganges sting ray (Himantura fluviatilis), and green sawfish (Pristis zijsron), which are of migratory nature and found in specific freshwater river systems. The large size of these fishes requires a specific type of crafts and gears for fishing and also helps in easy exclusion from the catch if caught accidently through traditional fishing methods.
Bill no. 159 proposed by MOEF & CC, Government of India as Wild Life (Protection) Amendment Act, 2021 recommended the first-time inclusion of three freshwater species, viz., O. belangeri (maximum length: 38 cm), S. semiplotus (maximum length: 60 cm), and P. denisonii (maximum length: 15 cm), endemic to rivers of Manipur, Assam, and WGs, respectively, as Schedule-I species. The wild stocks of all the three species have significantly reduced and are included in the red list of the IUCN as NT, VU, and EN, which warrant the implementation of measures to rebuild the stocks. If IUCN threatened status of wild stock of any fish species was the criteria for listing as Schedule-I species than the exclusion of so many other NT, EN and VU species (Table 3) appear unjustified. Most importantly, all these fishes are caught by local communities and fishermen as food fishes along with other freshwater fishes during the fishing activity. There are no fishing methods to exclude these fishes from the catch.
Bill no. 159 was subsequently reviewed by a Standing Committee on Science and Technology. The committee has revised the list of protected fish species (Report No. 365, 2022). The new list excludes O. belangeri, S. semiplotus, and P. denisonii, but recommended to include nine species, viz., golden mahseer (Tor putitora), Haragi (Hypselobarbus pulchellus), Bowany barb (Barbodes bovanicus), hump-backed mahseer (Tor remadeviae), loach (Mesonoemachelius herrei), loach (Schistura papulifera), catfish (Glyptothorax kudremukhensis), catfish (Glyptothorax kashmirensis), and Nilgiri mystus (Hemibagrus punctatus), under Schedule-I and seven species, viz., snakehead (C. aurantimaculata), rainbow snakehead (C. bleheri), Barca snakehead (C. barca), Canara pearl spot (E. canarensis), Maharaja barb (W. sahyadriensis), Zebra loach (B. striata), and Ocellated pufferfish (Leodon cutcutia), under Schedule-II. C. aurantimaculata and C. barca are indicated as DD in the IUCN list and L. cutcutia is LC while all the above 16 species are mentioned as “Not evaluated” under CITES (FishBase, 2022). All these fishes are again caught by local people and fishermen as food and few species to some extent are caught for aquarium trade and mainly export. The exclusion of P. denisonii, S. semiplotus, and O. belangeri was possibly based on the recommendation of Sanjy Molur, Executive Director, Zoo Outreach Organisation, Coimbatore, India (Report No. 365-Vol.II, Page no. 97; 2022), suggesting the exclusion of these fishes from Schedule-I as P. denisonii and S. semiplotus are netted with other food fishes, consumed by locals, and impossible to fish individually while O. belangeri has already become extinct from India. Molur and associates (Raghavan et al., 2013b) have been advocating restriction on fishing of P. denisonii and blaming aquarium trade for the depletion of the wild stock.
It is not important which fish species are excluded or granted protection under Wild Life Protection Act, 1972. The issue is on the inclusion of freshwater fish species that are widely distributed and caught by local people and fishermen as food, which cannot be excluded from netting even if desired. The local communities and fishermen will be catching the fish for self-consumption or for livelihood with the permission issued by the Department of Fisheries while the officials of the Department of Forest will be punishing and arresting them for violation of the law.
The imposing selective restriction on catching of threatened fishes specifically of freshwater origin with small to medium size and wide distribution may not be an effective strategy of endemic biodiversity conservation but through appropriate conservation measures (Arthington et al., 2016). The marking of “Freshwater Aquatic Biodiversity Reserves” could be one effective biodiversity conservation measure. These reserves will be much similar to Marine Protected Areas (MPA) established for the conservation of marine resources (Saravanan et al., 2011) or Fish Conservation Zones (FCZs) for freshwater fishes (Loury, 2020). The marking of FCZs proved to be highly successful in Cambodia, Lao PDR, Myanmar, Thailand, and Vietnam. The fishery of mahseer (Tor spp. and Neolissochilus strachery) in the Nago River of Thailand, Probarbus fishes (Probarbus jullieni and P. labeamajor), Irrawaddy dolphin (Orcaella brevirostris), and Mekong giant catfish (Pangasiandon gigas) in Mekong River, Lao PDR has been successfully improved through FCZs.
The “Freshwater Aquatic Biodiversity Reserves (FABRs)” will be small selective sections in multiple numbers of a large river both upstream and downstream. It will be a reserved zone with no commercial activity of any type, i.e., fishing, wood cutting, agriculture, and sand mining. Additionally, there will be a simultaneous effort to restore the habitat as per the specific requirement of the species to develop enormous breeding and feeding grounds for endemic aquatic species. It could be the construction of riffles and pools, the restoration of riparian vegetation, reviving the flow of water, etc. on select WGs rivers in the case of P. denisonii and other fishes with similar breeding and feeding habits. The marking of FABR will be an integrated approach of resource conservation and management through habitat restoration. The community participation (Adrianto et al., 2005) could be the key element of FABR in all stages from selection to management of sites as in case of FCZs.
A biodiversity conservation approach that will enhance the food availability and livelihood opportunities for local communities will be more effective than imposing complete restriction on resource utilization. There is continuous export of P. denisonii from India (Raghavan et al., 2013b), although the Government of Kerala has imposed a restriction on fish collection since 2008. Governments need to properly evaluate and frame a balanced policy to safeguard biodiversity as well as the socio-economic interest of local communities.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adrianto L., Matsuda Y., Sakuma Y. (2005). Assessing local sustainability of fisheries system: a multi-criteria participatory approach with the case of yoron island, Kagoshima prefecture, Japan. Mar. Policy 29 (1), 9–23. doi: 10.1016/j.marpol.2004.01.004
Agnew D. J., Pearce J., Pramod G., Peatman T., Watson R., Beddington J. R., et al. (2009). Estimating the worldwide extent of illegal fishing. PLoS One 4 (2), e4570. doi: 10.1371/journal.pone.0004570
Ali A., Raghavan R., Dahanukar N. (2015). Sahyadria denisonii. the IUCN red list of threatened species 2015. The IUCN Red List of Threatened Species 2015: e. T169662A7008246. doi: 10.2305/IUCN.UK.2015-1.RLTS.T169662A70082469.en
Anon (2006). Available at: http://www.practicalfishkeeping.co.uk/pfk/pages/show_article.php?article_id=714.
Anon (2020). “Handbook on fisheries statistics 2020,” in Department of fisheries, ministry of fisheries, animal husbandry and dairying, government of India (New Delhi, India: 196 p).
Arthington A. H., Dulvy N. K., Gladstone W., Winfield I. J. (2016). Fish conservation in freshwater and marine realms: status, threats and management. Aquat. Conservation: Mar. Freshw. Ecosyst. 26, 838–857. doi: 10.1002/aqc.2712
Arunkumar A. A., Manimekalan A. (2018). Freshwater fish fauna of rivers of the southern Western ghats, India. Earth System Sci. Data 10 (3), 1735–1752. doi: 10.5194/essd-10-1735-2018
Athira H., Kumar N. K. (2016). Scenario analysis of rice cultivation in kerala. J. Extension Educ. 28 (4), 5760–5763. doi: 10.26725/JEE.2016.4.28.5760-5763
Bartens J., Day S. D., Harris J. R., Dove J. E., Wynn T. M. (2008). Can urban tree roots improve infiltration through compacted subsoils for stormwater management? J. Environ. Qual. 37 (6), 2048–2057. doi: 10.2134/jeq2008.0117
Biju Kumar A., Smrithy R., Sureshkumar U., George S. (2015). Invasion of south American suckermouth armoured catfishes pterygoplichthys spp. (Loricariidae) in kerala, India-a case study. J. Threatened Taxa 7 (3), 6987–6995. doi: 10.11609/jott.1897.6987-6995
Biju C. R., Thomas K. R., Ajitkumar C. R. (2000). “Index areas selected for long time monitoring and conservation,” in Ecology of the hill streams of the Western ghats with special reference to fish community. final report, 1996-199). Eds. Chhapgar B. F., Manakadan R.(Bombay, India). Project report submitted to Bombay Natural History Society. 34–48.
Bill No. 159 (2021). The wild life (Protection) amendment bill 2021, a bill further to amend the wild life (Protection) act 1972, lok sabha, government of India 159. Available at: http://164.100.47.4/BillsTexts/LSBillTexts/Asintroduced/159_2021_ls_Eng.pdf
Braatne J. H., Rood S. B., Goater L. A., Blair C. L. (2008). Analyzing the impacts of dams on riparian ecosystems: a review of research strategies and their relevance to the snake river through hells canyon. Environ. Manage. 41 (2), 267–281. doi: 10.1007/s00267-007-9048-4
Braulik G. T., Wittich A., Macaulay J., Kasuga M., Gordon J., Gillespie D., et al. (2015). Fishing with explosives in Tanzania: Spatial distribution and hotspots. Wildlife Conserv. Soc. Tanzania Program Zanzibar 19. Available at: http://www.iucn-csg.org/wp-content/uploads/2010/08/WCS-Fishing-with-Explosives-Tanzania-Final-Report.pdf
Britz R., Adamson E., Raghavan R., Ali A., Dahanukar N. (2017). Channa pseudomarulius, a valid species of snakehead from the Western ghats region of peninsular India (Teleostei: Channidae), with comments on Ophicephalus grandinosus, O. theophrasti and O. leucopunctatus. Zootaxa 4299 (4), 529–545. doi: 10.11646/zootaxa.4299.4.4
Britz R., Anoop V. K., Dahanukar N., Raghavan R. (2019a). The subterranean Aenigmachanna gollum, a new genus and species of snakehead (Teleostei: Channidae) from kerala, south India. Zootaxa 4603 (2), 377–388. doi: 10.11646/zootaxa.4603.2.10
Britz R., Dahanukar N., Anoop V. K., Ali A. (2019b). Channa rara, a new species of snakehead fish from the Western ghats region of maharashtra, India (Teleostei: Labyrinthici: Channidae). Zootaxa 4683 (4), 589–600. doi: 10.11646/ZOOTAXA.4683.4.8
Brown A. V., Lyttle M. M., Brown K. B. (1998). Impacts of gravel mining on gravel bed streams. Trans. Am. fisheries Soc. 127 (6), 979–994. doi: 10.1577/1548-8659(1998)127<0979:IOGMOG>2.0.CO;2
Bukola D., Zaid A., Olalekan E. I., Falilu A. (2015). Consequences of anthropogenic activities on fish and the aquatic environment. Poultry Fisheries Wildlife Sci. 3 (2), 1–12. doi: 10.4172/2375-446X.1000138
Burrill B. A. (2014). Brown trout and their ecological impacts as an invasive species. Invasive Species Pacific Northwest 2014, 1–13.
Canonico G. C., Arthington A., McCrary J. K., Thieme M. L. (2005). The effects of introduced tilapias on native biodiversity. Aquat. Conservation: Mar. Freshw. Ecosyst. 15 (5), 463–483. doi: 10.1002/aqc.699
Dahanukar N., Raghavan R. (2013). Freshwater fishes of Western ghats: Checklist v1.0 august 2013. MIN, newsletter of the IUCN-SSC/WI freshwater fish specialist group south Asia & the freshwater fish conservation network of south Asia. 1, 6–16. Available at: https://www.zoosprint.zooreach.org/ZoosPrintNewsLetter/MIN_August_2013.pdf
Dahanukar N., Raghavan R., Ali A., Abraham R., Shaji C. P. (2011). “The status and distribution of freshwater fishes of the Western ghats,” in The status and distribution of freshwater biodiversity in the Western ghats, India. Eds. Molur S., Smith K. G., Daniel B. A., Darwall W. R. T. (Cambridge, UK and Gland, Switzerland: IUCN, and Coimbatore, India: Zoo Outreach Organisation), 21–48.
Dahanukar N., Raut R., Bhat A. (2004). Distribution, endemism and threat status of freshwater fishes in the Western ghats of India. J. biogeography 31 (1), 123–136. doi: 10.1046/j.0305-0270.2003.01016.x
Daniels R. J. R. (2011). Miss kerala in peril. Curr. Sci. 101 (12), 1518–1519. Available at: https://www.currentscience.ac.in/Volumes/101/12/1518.pdf
Day F. (1865). “The fishes of malabar,” in Bernard Quaritch (London: Bernard Quaritch Ltd), 293 p. doi: 10.5962/bhl.title.5549
de Klemm C., Shine C. (1993). Biological diversity conservation and the law (Switzerland and Cambridge, UK.: IUCN, Gland), xix + 292 p.
Dosskey M. G., Vidon P., Gurwick N. P., Allan C. J., Duval T. P., Lowrance R. (2010). The role of riparian vegetation in protecting and improving chemical water quality in streams. J. Am. Water Resour. Assoc. 46 (2), 261–277. doi: 10.1111/j.1752-1688.2010.00419.x
Dudgeon D. (2000). The ecology of tropical Asian rivers and streams in relation to biodiversity conservation. Annu. Rev. Ecol. Systematics 31 (1), 239–263. doi: 10.1146/annurev.ecolsys.31.1.239
Dynesius M., Nilsson C. (1994). Fragmentation and flow regulation of river systems in the northern third of the world. Science 266 (186), 753–762. doi: 10.1126/science.266.5186.753
Erarto F., Getahun A. (2020). Impacts of introduction of alien species with emphasis on fishes. Int. J. Fisheries Aquat. Stud. 8 (5), 207–216.
FAO, UNEP (2010). Report of the FAO/UNEP expert meeting on impacts of destructive fishing practices, unsustainable fishing, and illegal, unreported and unregulated (IUU) fishing on marine biodiversity and habitats, 23-25 September 2009, FAO fisheries and aquaculture report no. 932 Vol. 32 p (Rome: FAO). Available at: http://www.fao.org/docrep/012/i1490e/i1490e00.pdf.
FishBase (2022). A global information system on fishes, version: 02/2022. Available at: www.fishbase.se
Freedman J. A., Carline R. F., Stauffer J. R. Jr. (2013). Gravel dredging alters diversity and structure of riverine fish assemblages. Freshw. Biol. 58 (2), 261–274. doi: 10.1111/fwb.12056
Gadgil M. (2011). Reports of the Western ghats ecology expert panel. Ministry Environ. Forest Government India, 327. Available at: https://www.cppr.in/wp-content/uploads/2013/03/Gadgil-report.pdf
Guard M., Masaiganah M. (1997). Dynamite fishing in southern Tanzania, geographical variation, intensity of use and possible solutions. Mar. Pollut. Bull. 34, 758–762. doi: 10.1016/S0025-326X(97)00095-7
Gulati L. (1984). Fisherwomen on the kerala coast: Demographic and socio-economic impact of a fisheries development project, women, work and development Vol. 8) (Geneva: International Labour Organisation), 156 p.
Gunawardene N. R., Daniels A. E., Gunatilleke I. A. U. N., Gunatilleke C. V. S., Karunakaran P. V., Nayak K. G., et al. (2007). A brief overview of the Western ghats–Sri Lanka biodiversity hotspot. Curr. Sci. 93 (11), 1567–1572. Available at: https://www.currentscience.ac.in/Volumes/93/11/1567.pdf
Harvey B. C., Lisle T. E. (1998). Effects of suction dredging on streams: a review and an evaluation strategy. Fisheries 23 (8), 8–17. doi: 10.1577/1548-8446(1998)023<0008:EOSDOS>2.0.CO;2
Hyder A. B. K. (2003). Final project report “Riparian vegetation along the middle and lower zones of the chalakkudy river, kerala, India (Survey, mapping, community studies and identification of the residual pockets for conservation)”, project 26/2000 sponsored by kerala research programme on local level development (Thiruvananthapuram, Kerala: CDS), 118 p.
Hyder A. B. K., Devika M. A. (2020). Impact of dams on riparian vegetation: A case study in the chalakkudy river, Western ghats, India. J. Aquat. Biol. Fisheries 8, 7–13. Available at: http://keralamarinelife.in/Journals/Vol8-S/2-Bachan&Devika.pdf
Jayalal L., Ramachandran A. (2013). Major sustainability issues and comparative sustainability assessment of wild caught indigenous ornamental fishes exported from kerala, India. Fishery Technol. 50, 175–179. Available at: https://www.researchgate.net/publication/287331346
Jones J. R. E. (1964). Fish and river pollution Vol. 203 (Netherlands: Elsevier Publication), ISBN: (ISBN:9781483192482).
Joshi K. D., Basheer V. S., Kumar A., Srivastava S. M., Sahu V., Lal K. K. (2021). Alien fish species in open waters of India: Appearance, establishment and impacts. Indian J. Anim. Sci. 91 (3), 167–173.
Joshi K. D., Jha D. N., Alam M. A., Das S. C. S., Srivastava S. K., Kumar V. (2014). Massive invasion of resilient exotic fishes in the river ganga: A case study at allahabad stretch. J. Inland Fisheries Soc. 46 (1), 92–95. Available at: https://www.researchgate.net/publication/354545122
Jumani S., Rao S., Kelkar N., Machado S., Krishnaswamy J., Vaidyanathan S. (2018). Fish community responses to stream flow alterations and habitat modifications by small hydropower projects in the Western ghats biodiversity hotspot, India. Aquat. Conservation: Mar. Freshw. Ecosyst. 28 (4), 979–993. doi: 10.1002/aqc.2904
Katwate U., Jadhav S., Kumkar P., Raghavan R., Dahanukar N. (2016). Pethia sanjaymoluri, a new species of barb (Teleostei: Cyprinidae) from the northern Western ghats, India. J. Fish Biol. 88 (5), 2027–2050. doi: 10.1111/jfb.12980
Katwate U., Kumkar P., Raghavan R., Dahanukar N. (2020). Taxonomy and systematics of the 'Maharaja barbs' (Teleostei: Cyprinidae), with the description of a new genus and species from the Western ghats, India. Zootaxa 4803 (3), 544–556. doi: 10.11646/zootaxa.4803.3.9
Katwate U., Paingankar M. S., Raghavan R., Dahanukar N. (2014). Pethia longicauda, a new species of barb (Teleostei: Cyprinidae) from the northern Western ghats, India. Zootaxa 3846 (2), 235–248. doi: 10.11646/zootaxa.3846.2.4
Koehnken L., Rintoul M. (2018). Impacts of sand mining on ecosystem structure, process and biodiversity in rivers. World Wildlife Fund Int. 162. Available at: https://d2ouvy59p0dg6k.cloudfront.net/downloads/sand_mining_impacts_on_world_rivers__final_.pdf
Kottelat M., Whitten T. (1996). Freshwater biodiversity in Asia with special reference to fish. world bank technical paper no. 343 (Washington, D.C., USA.: The World Bank), 59 p. doi: 10.1596/0-8213-3808-0
Kulkarni A., Sabin T. P., Chowdary J. S., Koteswara Rao K., Priya P., Gandhi N., et al. (2020). “Precipitation changes in India,” in Assessment of climate change over the Indian region. Eds. Krishnan R., Sanjay J., Gnanaseelan C., Mujumdar M., Kulkarni A., Chakraborty S. (Singapore: Springer Nature Singapore Pte Ltd.), 47–72.
Kurup B. M., Harikrishnan M. (2008). “Population characteristics and stock assessment of puntius denisonii (Day) in kanzirapuzha river, kerala (South lndia),” in Ornamental fish breeding, farming and trade. Eds. Kurup B. M., Boopendranath M. R., Ravindran K., Banu S., Nair G. A. (Thiruvananthapuram, India: Department of Fisheries, Government of Kerala), pp.89–pp.98.
Kurup B. M., Radhakrishnan K. V. (2006a). Freshwater fish biodiversity of kerala; status and utilization for commercial fishing, food security and livelihood. Fishing Chimes 25 (10), 111–122.
Kurup B. M., Radhakrishnan K. V. (2006b). “Indigenous ornamental fish resources of Western ghats with special reference to kerala,” in Souvenir of ornamentals kerala 2006, international seminar on ornamental fish breeding, farming and trade, vol. 5-6. (Cochin, India: Department of Fisheries, Government of Kerala), 35–37.
Kurup B. M., Radhakrishnan K. V., Manojkumar T. G. (2004). “Biodiversity status of fishes inhabiting rivers of kerala (S. India) with special reference to endemism, threats and conservation measures,” in In proceedings of LARS2. 2nd large rivers symposium. Mekong river commission and food and agricultural organization (Rome: Food and Agriculture Organization), 163–182.
Lalmohan R. S., Rema Devi K. (2000). “Fish fauna of the chaliyar river, north kerala,” in Endemic fish diversity of Western ghats. Eds. Ponniah. A. G., Gopalakrishnan A. (Lucknow, UP, India: National Bureau of Fish Genetic Resources), pp.155–pp.156.
Latha A., Vasudevan M. (2016). State of india’s rivers for India rivers week. 59. Available at: indiariversblog.files.wordpress.com/2017/04/kerala-report.pdf
Lopez M. M., Gale P., Oidtmann B. C., Peeler E. J. (2010). Assessing the impact of climate change on disease emergence in freshwater fish in the united kingdom. Transboundary emerging Dis. 57 (5), 293–304. doi: 10.1111/j.1865-1682.2010.01150.x
Loury E. (2020). Establishing and managing freshwater fish conservation zones with communities: A guide based on lessons learned from critical ecosystem partnership fund grantees in the indo-Burma hotspot (Arlington, VA.: Critical Ecosystem Partnership Fund), 151 p. Available at: https://www.cepf.net/sites/default/files/fish-conservation-zone-guidebook.pdf.
Malik D. S., Sharma A. K., Sharma A. K., Thakur R., Sharma M. (2020). “A review on impact of water pollution on freshwater fish species and their aquatic environment,” in Advances in environmental pollution management: Wastewater impacts and treatment technologies, vol. Vol. 1 . Eds. Kumar V., Kamboj N., Payum T., Singh J., Kumar P. (Haridwar, India: Agro Environ Media, Publication Cell of Agriculture and Environmental Science Academy), 10–28. doi: 10.26832/aesa-2020-aepm-02
Marsh-Matthews E., Matthews W. J. (2000). Geographic, terrestrial and aquatic factors: which most influence the structure of stream fish assemblages in the midwestern united states? Ecol. Freshw. Fish 9 (1-2), 9–21. doi: 10.1034/j.1600-0633.2000.90103.x
Maulu S., Hasimuna O. J., Haambiya L. H., Monde C., Musuka C. G., Makorwa T. H., et al. (2021). Climate change effects on aquaculture production: sustainability implications, mitigation, and adaptations. Front. Sustain. Food Syst. 5. doi: 10.3389/fsufs.2021.609097
McManus J. W., Reyes R. B. Jr, Nanola C. L. J. Jr. (1997). Effects of some destructive fishing methods on coral cover and potential rates of recovery. Environ. Manage. 21 (1), 69–78. doi: 10.1007/s002679900006
Mercy T. V. A., Malika V. (2010). Final report of the project, (2007-2010) “Stock assessment and development of captive breeding technology of puntius denisonii (Day) - an indigenous ornamental fish of the Western ghats of India, marine products export development authority (Ministry of commerce and industry, government of India) (Conchin, India: A final project report submitted by authors to funding agency i.e., Marine Products Export Development Authority), 64 p.
Mercy T. V. A., Malika V., Sajan S. (2013). Reproductive biology of Puntius denisonii (Day 1865)-an endemic ornamental cyprinid of the Western ghats of India. Indian J. Fisheries 60 (2), 73–78.
Mercy T. V. A., Sajan S., Malika V. (2015). Captive breeding and developmental biology of Sahyadria denisonii (Day 1865) (Cyprinidae), an endangered fish of the Western ghats, India. Indian J. Fisheries 62 (2), 19–28.
Mercy T. V. A., Thomas K. R., Jacob E. (2002). Length-weight relationship of Puntius denisonii (Day). Indian J. Fisheries 49, 209–210.
Mingist M., Gebremedhin S. (2016). Could sand mining be a major threat for the declining endemic Labeo barbus species of lake tana, Ethiopia? Singapore J. Trop. Geogr. 37 (2), 195–208. doi: 10.1111/sjtg.12150
Mohite S. A., Samant J. S. (2013). Impact of environmental change on fish and fisheries in warna river basin, Western ghats, India. Int. Res. J. Environ. Sci. 2 (6), 61–70.
Molur S., Walker S. (1998). Report of the workshop “Conservation assessment and management plan for freshwater fishes of india” (Coimbatore, India: Zoo Outreach Organisation, Conservation Breeding Specialist Group, India), 156 p.
MPEDA. Marine products export development authority, ministry of commerce and industry, government of India, kochi, India. Available at: https://mpeda.gov.in/?page_id=1391.
Muhumuza M., Balkwill K. (2013). Factors affecting the success of conserving biodiversity in national parks: A review of case studies from africa". Int. J. Biodiversity 2013 20. doi: 10.1155/2013/798101
Myers N., Mittermeier R. A., Mittermeier C. G., Da Fonseca G. A., Kent J. (2000). Biodiversity hotspots for conservation priorities. Nature 403, 853–858. doi: 10.1038/35002501
Naiman R. J., Decamps H. (1997). The ecology of interfaces: riparian zones. Annu. Rev. Ecol. Systematics 28, 621–658. doi: 10.1146/annurev.ecolsys.28.1.621
Naiman R. J., Fetherston K. L., McKay S. J., Chen J. (1998). “Riparian forests,” in River ecology and management: Lessons from the pacific coastal ecoregion. Eds. Naiman R. J., Bilby R. E. (Berlin, Germany: Springer-Verlag), pp.289–pp.323. doi: 10.1007/978-1-4612-1652-0_12
Nair S. C. (1994). The high ranges: Problems & potential of a hill region in the southern Western ghats (New Delhi: Indian National Trust for Art & Cultural Heritage), 81p.
Nanditha J. S., van der Wiel K., Bhatia U., Stone D., Selton F., Mishra V. (2020). A seven-fold rise in the probability of exceeding the observed hottest summer in India in a 20C warmer world. Environ. Res. Lett. 15 (4), 1–13. doi: 10.1088/1748-9326/ab7555
Ngoan L. D. (2018). Effects of climate change in aquaculture: Case study in thua thien hue province, Vietnam. Biomed. J. Sci. Tech. Res. 10, 7551–7552. doi: 10.26717/BJSTR.2018.10.001892
Nilsson C., Reidy C. A., Dynesius M., Revenga C. (2005). Fragmentation and flow regulation of the world's large river systems. science 308 (5720), 405–408. doi: 10.1126/science.1107887
Owens P., Batalla R. J., Collins A. J., Gomez B., Hicks D. M., Horowitz A. J., et al. (2005). Fine-grained sediment in river systems: environmental significance and management issues. River Res. Appl. 21 (7), 693–717. doi: 10.1002/rra.878
Pankhurst N. W., Munday P. L. (2011). Effects of climate change on fish reproduction and early life history stages. Mar. Freshw. Res. 62 (9), 1015–1026. doi: 10.1071/MF10269
Paukert C., Schloesser J., Fischer J., Eitzmann J., Pitts K., Thornbrugh D. (2008). Effect of instream sand dredging on fish communities in the Kansas river USA: Current and historical perspectives. J. Freshw. Ecol. 23 (4), 623–633. doi: 10.1080/02705060.2008.9664250
Pethiyagoda R., Meegaskumbura M., Maduwage K. (2012). A synopsis of the south Asian fishes referred to Puntius (Pisces: Cyprinidae). Ichthyological Explor. Freshwaters 23 (1), 69–95.
Prasad G., Ali A., Raghavan R. (2008). Threatened fishes of the world: Puntius denisonii (Day 1865) (Cyprinidae). Environ. Biol. fishes 83 (2), 189–190. doi: 10.1007/s10641-007-9316-4
Pringle C. M., Freeman M. C., Freeman B. J. (2000). Regional effects of hydrologic alterations on riverine macrobiota in the new world: tropical-temperate comparisons. BioScience 50 (9), 807–823. doi: 10.1641/0006-3568(2000)050[0807:REOHAO]2.0.CO;2
Radhakrishnan K. V., Kurup B. M. (2005). “Aspects of life history traits of puntius denisonii (Day), an endemic and threatened ornamental fish of kerala,” in Sustain fish 2005. international symposium on sustainability of fish production systems and appropriate technologies for utilization (Kochi, India: Cochin University of Science and Technology). 16-18 march 2005(Abstract SAQ E18).
Radhakrishnan K. V., Kurup B. M., Murphy B. R., Xie S. G. (2012). Status of alien fish species in the Western ghats (India) as revealed from 2000–2004 surveys and literature analyses. J. Appl. Ichthyology 28 (5), 778–784. doi: 10.1111/j.1439-0426.2012.02029.x
Raghavan R., Dahanukar N., Krishnakumar K., Ali A., Solomon S., Ramprasanth M. R., et al. (2012). Western Ghats' fish fauna in peril: are pseudo conservationist attitudes to be blamed? Curr. Sci. 102 (6), 835–837.
Raghavan R., Dahanukar N., Tlusty M. F., Rhyne A. L., Kumar K. K., Molur S., et al. (2013b). Uncovering an obscure trade: threatened freshwater fishes and the aquarium pet markets. Biol. Conserv. 164, 158–169. doi: 10.1016/j.biocon.2013.04.019
Raghavan R., Philip S., Ali A., Dahanukar N. (2013a). Sahyadria, a new genus of barbs (Teleostei: Cyprinidae) from Western ghats of India. J. Threatened Taxa 5 (15), 4932–4938. doi: 10.11609/JoTT.o3673.4932-8
Raghavan R., Prasad G., Ali P. H., Pereira B. (2008). Fish fauna of chalakudy river, part of Western ghats biodiversity hotspot, kerala, India: patterns of distribution, threats and conservation needs. Biodiversity Conserv. 17 (13), 3119–3131. doi: 10.1007/s10531-007-9293-0
Raghavan R., Prasad G., Ali A., Pereira B., Baby F., Ramaprasanth M. (2010). 'Miss kerala' added to the IUCN red list of threatened species. Curr. Sci. 98 (2), 132–132.
Raghavan R., Prasad G., Ali P. A., Sujarittanonta L. (2007). Boom and bust fishery' in a biodiversity hotspot-is the Western ghats losing its most celebrated native ornamental fish, Puntius denisonii day? Curr. Sci. 92 (12), 1671–1672.
Raghavan R., Prasad G., Pereira B., Anwar Ali P. H., Sujarittanonta L. (2009). ‘Damsel in distress’-the tale of miss kerala, Puntius denisonii (Day), an endemic and endangered cyprinid of the Western ghats biodiversity hotspot (South India). Aquat. Conserve: Mar. Freshw. Ecosyst. 19, 67–74. doi: 10.1002/aqc.963
Ramachandra T. V., Subash Chandran M. D., Joshi N. V., Sreekantha, Saira V. K., Vishnu D. M. (2013). Influence of landscape dynamics on hydrological regime in central Western ghats-sahyadri conservation series 35, ENVIS technical report 65 (Bangalore, India: CES, Indian Institute of Science), 156 p.
Report No. 365 (2022)The wild life (Protection) amendment bill 2021 (Vol. II)-recommendations of the committee, government of India. Available at: https://rajyasabha.nic.in/rsnew/Committee_site/report_search.aspx.
Riegl B., Luke K. E. (1999). Ecological parameters of dynamited reefs in the northern red Sea and their relevance to reef rehabilitation. Mar. pollut. Bull. 37 (8-12), 488–498. doi: 10.1016/S0025-326X(99)00104-6
Rosenberg D. M., McCully P., Pringle C. M. (2000). Global-scale environmental effects of hydrological alterations: introduction. BioScience 50 (9), 746–751. doi: 10.1641/0006-3568(2000)050[0746:GSEEOH]2.0.CO;2
Saila S. B., Kocic V. L., McManus J. W. (1993). Modelling the effects of destructive fishing practices on tropical coral reefs. Mar. Ecol. Prog. Ser. 94 (1), 51–60. doi: 10.3354/meps094051
Saravanan K. R., Sivakumar K., Choudhury B. C. (2011). Status of marine and coastal environments and developing a marine protected area network in India (Dehradun, India: Wildlife Institute of India), 334 p.
Sekharan M., Ramachandran A. (2006). Threats in ornamental fish exports from India to Singapore. Seafood Export J. 2006, 11–17.
Shaji C. P., Easa P. S. (2001). Freshwater fishes of the Western ghats-a field guide (Lucknow, India: Kerala Forest Research Institute (KFRI), Peechi and National Bureau of Fish Genetic Resources (NBFGR).
Shaji C. P., Easa P. S., Gopalkrishnan A. (2000). “Freshwater fish biodiversity of Western ghats,” in Endemic fish diversity of Western ghats. Eds. Ponniah A. G., Gopalakrishnan A. (Lucknow, India: National Bureau of Fish Genetic Resources), pp 33–pp 55.
Shaji C. P., Laladhas K. P. (2013). Monsoon flood plain fishery and traditional fishing methods in thrissur district, kerala. Indian J. Traditional Knowledge 12 (1), 102–108.
Shelford V. E. (1918). Water pollution and fish life. J. Am. Water Works Assoc. 5 (4), 434–437. doi: 10.1002/j.1551-8833.1918.tb13190.x
Shyam S. S. (2020). Demand pattern and willingness to pay for high value fish consumption: Case study from selected coastal cities in kerala, south India. Indian J. Fisheries 67 (3), 135–143. doi: 10.21077/ijf.2020.67.3.70635-15
Shyam S. S., Rahman M. R., Antony B. (2015). Sardine economy of kerala: paradigms and perspectives. Int. J. Fisheries Aquat. Stud. 2 (6), 351–356.
Silas E. G., Gopalakrishnan A., Ramachandran A., Anna Mercy T. V., Sarkar K., Pushpangadan K. R., et al. (2011). Guidelines for green certification of freshwater ornamental fish MPEDA (Kochi, India: Marine Products Export Development Authority), 106 p.
Silva S. V. S., Dias A. H. C., Dutra E. S., Pavanin A. L., Morelli S., Pereira B. B. (2016). The impact of water pollution on fish species in southeast region of goiás , Brazil. J. Toxicol. Environ. Health A 79, 8–16. doi: 10.1080/15287394.2015.1099484
Singh G., Dukariya G. (2021). Insights in biodiversity management and conservation in India: Structure and role of multi-tier legal system. Asian J. Conserv. Biol. 10 (1), 40–45. doi: 10.53562/ajcb.AIRJ9111
Sudasinghe H., Dahanukar N., Raghavan R., Senavirathna T., Shewale D. J., Paingankar M. S., et al. (2021). Island colonization by a ‘rheophilic’fish: the phylogeography of Garra ceylonensis (Teleostei: Cyprinidae) in Sri Lanka. Biol. J. Linn. Soc. 132 (4), 872–893. doi: 10.1093/biolinnean/blaa221
Sugunan V. V. (1995). Reservoir fisheries of india. FAO fisheries technical paper 345 (Rome: FAO), 423 p.
Suhair K., Kumar S. (2015). A handbook on induced breeding technology and larval rearing of ornamental fish “Miss kerala” sahyadria denisonii (Day 1865) (Government of Kerala: Department of Fisheries), 32 p.
Tabacchi A. M. P., Tabacchi E., Naiman R. J., Deferrari C., Decamps H. (1996). Invasibility of species-rich communities in riparian zones. Conserv. Biol. 10 (2), 598–607. doi: 10.1046/j.1523-1739.1996.10020598.x
Talbot R. (2013). India’s underground fish trade. Amazonas Magazine 2013. Available at: https://www.amazonasmagazine.com/2013/06/28/indias-underground-fish-trade/
Talwar P. K., Jhingran A. G. (1991). Inland fishes of India and adjacent countries (Vol.1 & 2) (New Delhi: Oxford-IBH Publishing Co. Pvt. Ltd.), 1158 p.
Thampy D. R., Sethu M. R., Paul M. B., Shaji C. P. (2021). Ichthyofaunal diversity in the upper-catchment of kabini river in wayanad part of Western ghats, India. J. Threatened Taxa 13 (2), 17651–17669. doi: 10.11609/jott.6159.13.2.17651-17669
Thomas R. (2010). Habitat and distribution of hill stream fishes of southern kerala (south of palghat gap) (Kottayam, Kerala, India: Department of Zoology, Mahatma Gandhi University), 240 p. PhD Thesis.
Thomas J. J. (2011). Paddy cultivation in kerala. AgEcon Research Rev. Agrarian Stud. 1 (2), 215–226.
Trend Economy (2020). World merchandise exports and imports by commodity. Available at: www.trendeconomy.com
United Nations Environment Programme (2018). Law and national biodiversity strategies and action plans (Nairobi, Kenya.: United Nations Environment Program), 90 p.
Vaughan A. (2021) Ten conservation success stories when species came back from the brink. Available at: https://www.newscientist.com/article/mg24933223-400-ten-conservation-success-stories-when-species-came-back-from-the-brink/.
Keywords: Puntius denisoni, Sahyadria denisonii, Densison barb, redline torpedo barb, Western Ghats, fish biodiversity, monsoon flood plains fishery, Freshwater aquatic biodiversity reserves
Citation: Jain AK, Mercy TVA and Jain A (2022) Issues on the inclusion of Puntius denisonii (Day), a freshwater ornamental fish of global value, as Schedule-I species under the Wild Life (Protection) Amendment Act, 2021 of India. Front. Mar. Sci. 9:944680. doi: 10.3389/fmars.2022.944680
Received: 15 May 2022; Accepted: 10 August 2022;
Published: 08 September 2022.
Edited by:
Shubhadeep Ghosh, Pelagic Fisheries Division (ICAR), IndiaReviewed by:
Supratim Chowdhury, West Bengal University of Animal and Fishery Sciences, IndiaVeliyil Vasu Sugunan, Indian Council of Agricultural Research (ICAR), India
Debabrata Panda, Central Institute of Freshwater Aquaculture (ICAR), India
Copyright © 2022 Jain, Mercy and Jain. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Atul Kumar Jain, atulsalinewater@yahoo.co.in
 Atul Kumar Jain
Atul Kumar Jain T. V. A. Mercy
T. V. A. Mercy Abhinika Jain
Abhinika Jain