Neogobius fluviatilis Ponticola kessleri
Neogobius fluviatilis Ponticola kessleri
Neogobius fluviatilis Ponticola kessleri
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
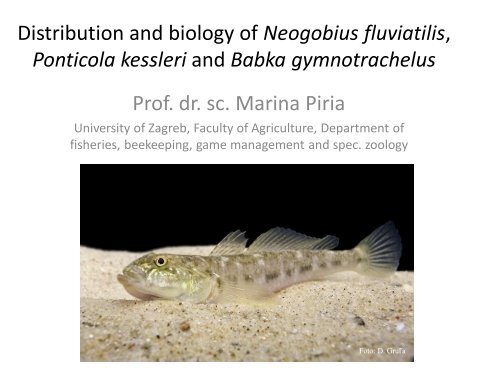
Distribution and biology of <strong>Neogobius</strong> <strong>fluviatilis</strong>,<br />
<strong>Ponticola</strong> <strong>kessleri</strong> and Babka gymnotrachelus<br />
Prof. dr. sc. Marina Piria<br />
University of Zagreb, Faculty of Agriculture, Department of<br />
fisheries, beekeeping, game management and spec. zoology
<strong>Neogobius</strong> <strong>fluviatilis</strong> (Pallas, 1814) – monkey goby<br />
- second dorsal fin ray (first branched ray about twice as long as<br />
penultimate ray),<br />
- nape scale structure,<br />
- pelvic disc fraenum with small rounded lobes<br />
- scales in midlateral series<br />
- First dorsal without black spot<br />
<strong>Ponticola</strong> <strong>kessleri</strong> (Günther, 1861) – bighead goby<br />
- second dorsal fin ray (first branched ray about as long as<br />
penultimate ray)<br />
- Nape complitely covered with ctenoid scales<br />
- pelvic disc fraenum with angular lobes<br />
- First dorsal without black spot<br />
Babka gymnotrachelus (Kessler, 1857) – racer goby<br />
- irregular position and shape of diagonal bars on body;<br />
- first branched ray of second dorsal about as long as penultimate<br />
ray;<br />
- no scales on midline of nape, in front of preoperculum;<br />
- pelvic-disc fraenum with small rounded lobes<br />
- first dorsal without black spot
The sampling efficiency of electrofishing for<br />
<strong>Neogobius</strong> species<br />
• Reyol et al. (2005)- estimetad electrofishing efficiency 55% in<br />
rock dominated habitats – for all bentic fishes<br />
• Wiesner (2004)- estimetad electrofishing efficiency for<br />
<strong>Neogobius</strong> spp. under lab. conditions (artificial rip rap<br />
analogy)- 30-50%<br />
(Polačik et al., 2008)<br />
• Single pass electrofishing is commonly used in large rivers,<br />
and this method is frequently used to sample <strong>Neogobius</strong> spp.<br />
In areas with rocky substrate
Aim of investigation<br />
• To determine the efficiency of single pass<br />
electrofishing in areas with rocky substrate<br />
• Hypotesis: expected lower efficiency of<br />
electrofishing for these bentic species in their<br />
natural riverine environment
Material and methods<br />
• Fish was captured at main<br />
channel of the Danube<br />
River in the town of Vidin<br />
Bulgaria<br />
• Backpach unit Lena, pulsed<br />
DC, 85Hz, 300 V with<br />
eliptical stainless steel<br />
anode 40x20 cm and 4 mm<br />
mesh size; 0.6 m long<br />
stripe shaped copper<br />
cathode
Results<br />
Species Mean efficiency<br />
N. Fluviatilis 35.4<br />
B. Gymnotrachelus 50.1<br />
P. Kessleri 16.7<br />
N. melanostomus 23.7<br />
<strong>Neogobius</strong> spp. 29.7<br />
Percids 74.6
• Backpack and towboat electrofishing can be used to<br />
consistently capture N. melanostomus in clear, wadeable<br />
water, although this method requires the operator to focus<br />
on searching for fish on the bottom and may be aided by<br />
overturning rocks<br />
• N. elanostomus lacks a swimbladder and does not float<br />
when electrofished (Phillips et al., 2003; Kornis & Vander<br />
Zanden, 2010).<br />
• Beach seining is inefficient at sampling the preferred rocky<br />
habitat, but kick seining, whereby a short (c. 2 m) seine is<br />
placed downstream of rocky habitat to collect fish as the<br />
rocky habitat is disturbed by kicking, has proven effective<br />
(Jude et al., 1995)
N. <strong>fluviatilis</strong><br />
• The natural habitat of the monkey<br />
goby is the fresh and brackish waters<br />
of basins in the Black Sea and the Sea<br />
of Marmara.<br />
• In the Black Sea and the surrounding<br />
areas, the monkey goby is common in<br />
all desalinated water including the<br />
Danube river and its tributaries, the<br />
lagoons and estuaries of the northwestern<br />
part of the Black Sea, the Sea<br />
of Azov, and the rivers of Caucasus.<br />
Kottelat and Freyhof, 2007
• Recently, the monkey goby has been registered as<br />
an invasive species in some countries of Europe.<br />
• In 1970, the species was first declared as a nonindigenous<br />
in Lake Balaton in Hungary<br />
• discovered in the Middle Danube in Hungary in<br />
1984.<br />
• In 2001 it was found to have spread to the Slovak-<br />
Hungarian sector of the Danube River.<br />
• In the basin of the Baltic Sea it was first registered<br />
as an invasive species in 1997.<br />
• The species has also become a common sight in the<br />
Włocławek Reservoir and Zegrze Reservoir (Poland).<br />
• has been found in the German part of the river<br />
Rhine since March 2009.<br />
• It has also been found in the Waal River, near<br />
Nijmegen, the Netherlands<br />
• In August 2011 the monkey goby is registered for<br />
the first time in the Evros River (Greece), which is<br />
inflows to the Aegean Sea<br />
• In Croatia – Danube River, Sava river and its<br />
tributaries
Biology<br />
• Occurs in inshore habitats, estuaries and<br />
brackish- and fresh-water lagoons and lakes; large<br />
to medium sized rivers and streams; on sand or<br />
mud bottom<br />
• It is one of the most abundant fish in lowland<br />
rivers.<br />
• This species lives up to 5 years; The average adult<br />
monkey goby measures 7–10 centimeters, but<br />
has been known to grow to lengths of 18–20<br />
centimeters
Spawning<br />
• spawns for the first time at 2 years;<br />
• spawning season in April to July, locally until<br />
September, when temperature is above 13°C<br />
• females may repeat spawning during a season.<br />
• Males with body completely black with yellow fin<br />
margins during the spawning season;<br />
• these excavate nests under any kind of hard<br />
substrate and guard eggs until hatching;<br />
• with adhesive eggs deposited on stones, shells<br />
and aquatic plants
Feeding<br />
• the diet of the monkey goby consists of amphipods, molluscs, and Oligochaeta<br />
Joanna Grabowska, Michał Grabowski, Anna Kostecka (2009):<br />
Diet and feeding habits of monkey goby (<strong>Neogobius</strong> <strong>fluviatilis</strong>) in a newly invaded area<br />
- how this new species influences native freshwater<br />
communities especially when the invader is a<br />
predator such as the monkey goby? One impact may<br />
be the reduction in size of the populations of<br />
indigenous prey species<br />
Fig. 1 Sampling site locations. The black<br />
line and arrow indicate the current range<br />
and direction of expansion of the monkey<br />
goby in the Vistula River system
• In the majority of habitats in its natural range, both marine and freshwater<br />
monkey goby were significant mollusc consumers, although a wide range<br />
of prey were observed, based chiefly on macroinvertebrates (annelids,<br />
crustaceans, insects) rather than on fish (see Pinchuk et al. 2003 for<br />
review).<br />
• An ontogenic, seasonal and geographical variation in diet was observed<br />
(see Pinchuk et al. 2003 for review).<br />
• The broad spectrum of diet described for the species in its natural range<br />
may be a crucial factor for its success in invading new territories.<br />
• The information on the monkey goby diet outside its natural range is very<br />
limited (Biro´ 1995; Kakareko et al. 2005).<br />
• The purpose of our study was to define the monkey goby diet spectrum,<br />
feeding preferences, spatial and size related changes in diet, its diurnal<br />
feeding activity, as well as to predict which groups of native preys would<br />
be most affected by the presence of this exotic predator in a newly<br />
invaded location such as the Vistula River system
• The fish consumed<br />
insect larvae and pupae,<br />
crustaceans, annelids,<br />
gastropods and fish<br />
• Chironomid larvae were<br />
a prevalent food<br />
category in all sampling<br />
sites, followed by<br />
amphipod crustaceans<br />
at one site and by<br />
trichopteran larvae and<br />
chironomid pupae at<br />
another
Food<br />
• According to the values of the<br />
Ivlev’s selectivity index, the<br />
preferred food category were<br />
chironomid larvae.<br />
• No significant differences in diet<br />
were found over the 24-h cycle.<br />
• There was no variation among<br />
different fish size groups.<br />
• the species, due to its ability to<br />
use locally available food<br />
resources, displays a<br />
generalistic and highly flexible<br />
feeding strategy.
M. Polačik, M. Janač1,2, P. Jurajda1, Z. Adamek1, M. Ondračkova, T. Trichkova, M. Vassilev (2008): Invasive<br />
gobies in the Danube: how do they utilize the new environment?<br />
In addition to the<br />
Austrian and Bulgarian stretch, the<br />
Croatian stretch of the Danube<br />
(which is the non-native<br />
range of <strong>Neogobius</strong> spp., was<br />
sampled in early November 2005<br />
Fig. 4. The Costello’s plots of<br />
monkey goby (a), racer goby<br />
(b), bighead goby (c) and<br />
round goby (d). The prey<br />
items consumed by native<br />
populations are denoted by<br />
circles and those consumed<br />
by the non-native populations<br />
by triangles. The prey items of<br />
low prey – specific<br />
importance (< 0.2) and<br />
consumed with low frequency<br />
(< 20 %) were omitted from<br />
the plots. NCBDL = Nonchironomid<br />
benthic Dipteran<br />
larvae.
Maria Capova, Ivana Zlatnicka, Vladimır Kovac<br />
Stanislav Katina (2008): Ontogenetic variability in the external morphology<br />
of monkey goby, <strong>Neogobius</strong> <strong>fluviatilis</strong> (Pallas, 1814)<br />
and its relevance to invasion potential<br />
• Monkey goby has significantly smaller lower jaw length<br />
and maxilla length than the round and bighead gobies,<br />
which indicates a smaller mouth gap.<br />
• This demonstrates specialization of monkey goby on<br />
smaller diet items compared to both round and<br />
bighead gobies.<br />
• the diet of monkey goby in Slovakia is composed<br />
mainly from Chironomid larvae and small Crustaceans<br />
• the prey of bighead and round gobies also include<br />
bigger items, such as Molluscs, Amphipods and<br />
Trichoptera larvae, with bighead goby preying even on<br />
small fishes (Kosco et al., 2006; Adamek et al., 2007).
• Monkey goby reach their definite phenotype very early in their ontogeny<br />
and thus represent a strongly precocial (=specialized) species with direct<br />
development (Flegler-Balon, 1989).<br />
• The morphological differences between monkey goby and the two other<br />
goby species discussed in the present study also refer to its strong<br />
specialization to sandy substrata and smaller prey.<br />
• This corroborates previously reported data on habitat and items<br />
preferences of monkey goby (Berg, 1949; Kazancheev, 1963; Svetovidov,<br />
1964; Eros et al., 2005; Kosco et al., 2006; Adamek et al., 2007).<br />
• Therefore, it can be expected that monkey goby should not spread to new<br />
areas as fast as round and bighead gobies and if they do, they should be<br />
limited to habitats with sandy and/or sandy gravel bottom.<br />
• If this assumption is correct, then it follows that the potential impact of<br />
monkey goby on native fauna or even ecosystem is likely not to be as<br />
negative as it can be in the case of bighead and round gobies.
P. <strong>kessleri</strong><br />
• It is present in lagoons and estuaries<br />
of the north-western Black Sea, near<br />
the Bulgarian coast, especially in<br />
lakes Mandra, Vaya, Varna,<br />
Beloslavsko<br />
• In the Danube River the original<br />
distribution of the bighead goby<br />
reached Vidin and was common in<br />
the lakes of the Danube delta.<br />
• It inhabits the rivers Dniester up to<br />
Kamianets-Podilskyi, small rivers<br />
Zbruch and Bystrytsia.<br />
• Dnieper up to Dnipropetrovsk, also in<br />
the Southern Bug River.
• The bighead goby was recorded as<br />
non-indigenous species in the Slovak<br />
section of the Danube River in 1996<br />
and until 2004, this species had the<br />
widest density and distribution among<br />
the four Gobiidae species.<br />
• In the Danube River basin, species is<br />
also mentioned as non-indigenous in<br />
the Tisza River.<br />
• In the Upper Danube it was registered<br />
in Austrian and German parts up to the<br />
City of Straubing<br />
• During 2000-2002 - registered in small<br />
streams of the Black Sea coast of<br />
Eastern Turkey<br />
• Since March 2009 the fish is registered<br />
in the North Sea basin, in the Waal<br />
River, the Netherlands<br />
• At the German part of the Lower<br />
Rhine, between the cities of Cologne<br />
and Rees, this species consists of 52%<br />
of gobies catchments in 2009
• Found usually in freshwater and brackish water<br />
with very low salinity (< 2 ppt); in lower rivers<br />
and lagoons, lakes, large rivers, harbours on rocky<br />
or well-vegetated bottom (reed thickets) in still<br />
waters as well as rapids.<br />
• Initial spawning at 2 years, in March to May,<br />
where adhesive eggs are deposited on stones,<br />
shells and aquatic plants; while males guard the<br />
eggs until hatching.<br />
• Feeds on crustaceans (mysids, corophiid<br />
amphipods) and small fish (gobies)
The prey items consumed by native populations are denoted by circles<br />
and those consumed by the non-native populations by triangles.
Babka gymnotrachelus<br />
• This goby inhabits the coasts of Turkey, the<br />
rivers of the Caucasus, including Inguri,<br />
Rioni and the rivers of Kolkhida, including<br />
Lake Paliastomi, Lake Suzha.<br />
• In the north-western Black Sea it inhabits the<br />
Dnieper-Bug Estuary, Dniester Estuary, near<br />
the Tendra sandbar and Berezan Island.<br />
• In the Danube River it is widspread up to<br />
Vidin, and lives in tributaries and lakes of<br />
Danube delta, including Brateş, Kahul,<br />
Yalpug, Katlabuh, Kitay, Razelm, etc.<br />
• It inhabits the Dniester River and its<br />
tributaries, including Zbruch, Zhvanchik,<br />
Smotrych, Răut, Bîc, Dubăsari Reservoir.<br />
• It is common in the Southern Bug River and<br />
in the Dnieper River as far as Kiev. It lives in<br />
the Kamchiya River and Lake Shablensko in<br />
Bulgaria.<br />
• In the Sea of Azov it is in Taganrog Bay and<br />
the Don, Aksay, Seversky Donets rivers.<br />
• Also inhabits the Caspian Sea, where<br />
presented by the subspecies Babka<br />
gymnotrachelus macrophthalmus.
• Occurs in brackish- and fresh-water habitats with low<br />
salinity (< 2 ppt); lagoons and lakes; large rivers to small,<br />
fast-flowing streams; on sand or mud bottom; mainly in<br />
well vegetated or high-complexity habitats.<br />
• Abundant in backwaters and still channels<br />
• Longevity is 4-5 years; spawns for the first time at 2 years;<br />
spawning season in April to June, occasionally until mid-<br />
August; females may repeat spawning during a season;<br />
usually spawns for a single season.<br />
• Males guard eggs until hatching; with adhesive eggs<br />
deposited on stones, shells and aquatic plants<br />
• Feeds on crustaceans (esp. Corophiid amphipods), aquatic<br />
insects (mostly chironomid larvae), polychaetes, also small<br />
fish and mollusks
• Amphipods constituted 11–70% of<br />
total gut content biomass and<br />
were found on average in 84% of<br />
analysed alimentary tracts.<br />
• The second prey types were<br />
chironomid larvae (16–63% of<br />
total food biomass; frequency<br />
occurrence: 61–91%), and to a<br />
lesser extent chironomid pupa,<br />
ceratopogonid larvae, oligochaets,<br />
dipteran imagines and copepods,<br />
with fish larvae found in the gut of<br />
eight gobies.<br />
• racer goby forages mainly on<br />
benthos and has a nocturnal<br />
feeding activity.<br />
(2005)
T. Kakareko, J. Z_ bikowski and J. Z_ ytkowicz (2005): Diet partitioning in summer of two syntopic neogobiids<br />
from two different habitats of the lower Vistula River, Poland<br />
- the diet of <strong>Neogobius</strong> species in the lower Vistula varied according to prey<br />
availability, which itself was associated to the character of the habitat from<br />
which the fish were taken.
Thank you
• In successive years its expansion seemingly stopped, but in 1993 its<br />
explosion-like propagation was observed in a reservoir constructed<br />
at the middle section of River Tisza („Tisza-tó” reservoir), as earlier<br />
in Lake Balaton (Harka 1993), respectively.<br />
• That time this appeared as isolated habitat, but later on it was<br />
discovered that the species was present in both the lower and<br />
middle reaches of the river in Serbia and Hungary (Guelmino 1994,<br />
Györe et al. 2001).<br />
• During the last decade the monkey goby expands in rivulets flowing<br />
into L. Balaton (Bíró & Paulovits 1994, Bíró et al. 2002), in the<br />
Hungarian-Croatian border-section and in River Tisza and its side<br />
rivers (Sallai 2002).<br />
• In River Danube, the species moves upstream: in 2001 it was<br />
collected at the Hungarian-Slovak section (Stráňai & Andreji 2001;<br />
Sallai 2003; Holčík et al. 2003), and in 2003 it was caught at the<br />
lower section of R. Raba, near to R. Danube and the Austrian border<br />
(G. Guti personal communication).